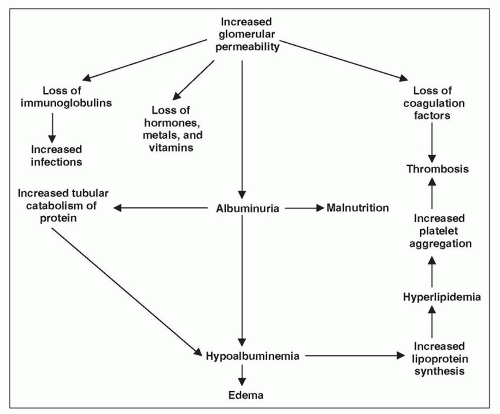
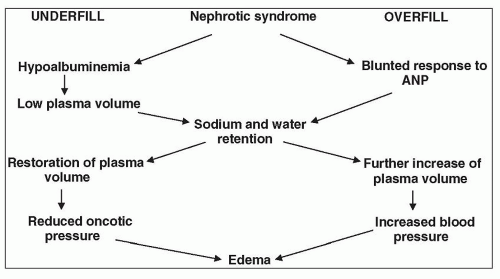
Because the major characteristic of the NS is heavy proteinuria, it would be helpful at this point to review our understanding of the filtration of macromolecules.
Brief Review of Anatomy
The anatomy of the glomerular capillary wall is discussed in detail in Chapter 1, and only the salient points are emphasized here. Traditionally, the glomerular filter has been considered to be composed of three components: the endothelial layer of the glomerular capillary, the glomerular basement membrane (GBM), and the podocytes with their interdigitating foot processes and slit diaphragms. More recently, two additional components, namely, the glycocalyx of the endothelial cells and the subpodocyte space (SPS), have been found to contribute to
glomerular permselectivity (
2,
3). Starting on the luminal side and proceeding outward, the glomerular capillary wall consists of the following structures. First, there is a fenestrated endothelial cell layer with a negatively charged glycocalyx coat. The glycocalyx is composed of proteoglycans with a core of perlecan, syndecan, and versican and covalently bonded side chains of glycosaminoglycans such as heparan sulfate (
3). Various serum proteins may become adsorbed to this surface. The endothelial fenestrations occupy between 20% and 50% of the endothelial surface and are maintained by vascular endothelial growth factor (VEGF) secreted by the podocytes (
3,
4). The basement membrane can be resolved into three layers: the lamina rara interna, the lamina densa, and the lamina rara externa. The GBM is composed of type IV collagen (with a triple helix of α3, α4, and α5 chains) forming a three-dimensional framework that serves as a lattice for the remaining components (
2,
5). Laminin that is bound to the collagen by entactin and nidogen anchors both endothelial and epithelial cells via the α
3β
1-integrin (
2,
6,
7) and contributes additional structural support to the collagen. The negative charge of the GBM is imparted by the heparan sulfate proteoglycans, primarily perlecan and agrin (
2,
7,
8).
The podocyte or visceral epithelial cell is recognized as a major player in glomerular filtration. The foot processes—which interdigitate—are attached to the outer aspect of the GBM by both laminin and fibronectin (
5). The dystroglycan complex links α
3β
1-integrin to the actin cytoskeleton in the foot processes, and its proper glycosylation is important in maintaining the foot processes (
9). Additional proteins involved in the development and maintenance of the foot processes include podocalyxin, podoplanin, glomerular epithelial protein 1 (GLEPP-1), and glucocorticoid-induced transcript 1 (
7,
10,
11). MicroRNAs also play a role in podocyte function (
12). Synaptopodin and actin are important in podocyte motility, and megalin plays a role in endocytosis at the base of the foot processes (
7). Chloride intracellular protein 5 may act as an adapter between the actin cytoskeleton and the plasma membrane of the podocyte (
13). Filtration slits are located between adjacent foot processes and are bridged by the slit diaphragm. Since the description of nephrin in 1998 (
14), more than 15 different gene products have been localized to or near the slit diaphragm (
2,
15,
16). These proteins also help maintain the shape of the foot processes, are important in signaling pathways, and function in regulation of cytoskeleton, polarized sorting and endocytosis, cell differentiation, suppression of differentiation, mechanotransduction, and podocyte viability (
15). The exact shape and size of the pores in the slit diaphragm are not known. The original description posited a zipper-like structure (
17). A more recent study suggests the possibility of a heteroporous structure (
18). The anatomy of the podocyte and the slit diaphragm is more fully described in Chapter 1. Three-dimensional reconstruction has demonstrated the presence of a flow-restrictive SPS (
3). This is discussed below in the section on the effect of hemodynamic factors.
Factors Involved in Glomerular Filtration
Numerous factors control the filtration of macromolecules, and they are best considered under the following headings: the various properties of the molecules themselves, the principal ones being size, charge, and shape; the properties of the capillary wall; and hemodynamic factors, chief of which are the glomerular plasma flow rate and transcapillary hydraulic pressure difference.
Properties of Molecules
Fractional clearance studies have determined how size affects the ability of molecules to cross the glomerular filter. When tritiated neutral dextrans were used to test glomerular permeability in the rat, their fractional clearance was 1 (no measurable restriction of filtration) when the effective hydrodynamic radius,
ae, was ≤20 Å; but with increasing
ae values, the fractional clearance decreased progressively until it approached 0 with radii greater than approximately 40 Å (
19,
20). Numerous studies over the years have confirmed that for neutral solutes, the sieving coefficient decreases with increasing molecular size (
21). Charge also plays a role in controlling the transglomerular passage of macromolecules as suggested by the observation of Michael et al. (
22) that there was a reduction in negative charge in the glomerular capillaries associated with proteinuria in rats with aminonucleoside nephrosis and in humans with MCD. Since these initial observations were made, fractional clearance studies using different sizes of neutral dextran and comparing their fractional clearances with those of negatively charged dextran sulfate of similar size and cationic dextran molecules supported the importance of electrostatic charge (
23,
24). However, others have argued that there is no charge selectivity and that negatively charged molecules of small size such as albumin may pass freely through the glomerulus to be reabsorbed by tubules (
25). A raging controversy developed that is discussed below in the context of the properties of the capillary wall. Shape of the molecule undergoing filtration is another important determinant of filtration (
26,
27).
Properties of the Capillary Wall
The various elements of the glomerular capillary wall act together in series to control the filtration of macromolecules forming both size and charge barriers. The barrier is dynamic with cross talk between the epithelial and endothelial cells (
28,
29). The size-selective barrier resides in all layers of the glomerular filtration barrier. Originally, the GBM was thought to be the principal size-selective barrier (
7), but this is no longer the prevailing opinion (
29). Nonetheless, the occurrence of proteinuria associated with mutations in laminin (a protein found only in GBM) supports a contribution of the GBM to the size selectivity of the barrier (
29). The role of the filtration slit diaphragm, first described by Rodewald and Karnovsky (
17), was confirmed by Kestila et al. (
14) following their discovery of nephrin mutations in congenital nephrotic syndrome (CNS) of the Finnish type in association with a loss of the slit diaphragms. More recent work shows a heteroporous structure in the slit diaphragms with variation in the size of the pores under pathologic conditions (
18). Edwards et al. found that the sieving curve was highly dependent on changes in filtration slit width and not in changes in the GBM (
30). They concluded that size selectivity is most sensitive to the size of the slit diaphragm and the hindrance coefficient of the GBM (
30). Most recently, a role for the endothelial surface layer has also been described for size restriction (
31). This layer is composed of glycocalyx composed of proteoglycans bound to the cell membrane and an endothelial cell coat attached to the glycocalyx. This cell coat consists of proteoglycans, glycosaminoglycans, glycoproteins, and various plasma proteins such as
albumin and orosomucoid (
31). Deen (
32) using mathematical modeling suggested that the endothelial and epithelial cell layers are most important for size selectivity.
Originally, it was believed that the charge barrier resided chiefly in the anionic sites of the GBM (
33). It is now generally accepted that the charge barrier also resides in the endothelial and/or epithelial cells (
7,
21,
28,
29). Jeansson and Haraldsson (
34) gave hyaluronidase and heparitinase III to mice and measured glomerular selectivity in both in vivo and isolated perfused kidneys. They found evidence for both size and charge selectivity using a heterogeneous charged fiber model. Treatment with hyaluronidase increased albumin permeability fourfold without changing the selectivity of similar sized neutral molecules. Additional evidence for the presence of both size and charge barriers was obtained from a study of adriamycin-induced NS in the mouse. The investigators demonstrated increased clearance of larger Ficolls in treated mice as compared to control mice (
35). Furthermore, loss of charge selectivity was also established by comparison of the clearance of neutral as compared to anionic albumin (
35). These changes were associated with a thinning of the glycocalyx of the endothelial cells and down-regulation of synthesis of the heparan sulfate proteoglycans (
35). However, it has also been suggested that no charge barrier exists. Some investigators believe that albumin passes through the capillary wall but is then returned to the plasma by a proximal tubular albumin retrieval pathway in which megalin, a low-affinity albumin-binding receptor, directs albumin to lysosomes or via transcytosis to the basolateral membrane and cubilin, a higher-affinity albumin receptor, directs albumin to lysosomes (
25). These authors suggest that proteinuria is due to inhibition of this retrieval pathway rather than increased glomerular permeability. This group using in vivo two-photon microscopy demonstrated that the glomerular sieving coefficient for albumin was higher than expected to a degree that supported lack of a charge barrier (
36). This raised a storm of controversy with contentious articles and editorials. Several other investigators using the same two-photon technique demonstrated a glomerular sieving coefficient for albumin that was close to or at the expected value (
37,
38) supporting the presence of a charge-selective barrier. Most recently, Sandoval et al. (
39) have shown that the glomerular sieving coefficient may vary in different strains of rats and under different conditions. Thus, the controversy is not yet entirely resolved and requires further consideration as our understanding evolves (
40). Nonetheless, most investigators agree that a charge-selective barrier is present in the glomerulus. Additional evidence for the presence of a glomerular charge barrier is supported by the association of proteinuria with glomerular injury particularly the accumulating evidence of mutations to various elements of the glomerular filter and the occurrence of proteinuria (
28).
Hemodynamic and Other Biophysical Factors
Hemodynamic factors, including blood flow, convection, diffusion, transcapillary hydraulic pressure difference, and intra-glomerular pressure, have been shown to play an important role in the passage of macromolecules across the glomerulus (
19). Fractional clearance of a solute depends on the relative transport of water and the molecule in question. The latter takes place by both convection and diffusion for intermediate-sized macromolecules (an
ae value between approximately 20 and 34 Å). For small and large macromolecules, convection is the predominant mode of solute transport. An increase in the glomerular filtration rate (GFR) results in comparable increments in water flux and solute transport by convection so that no change in fractional clearance occurs when diffusion is not a factor. However, in the case of intermediate-sized molecules, an increase in GFR produces a decrease in diffusion and, therefore, a reduction of fractional clearance. Afferent arteriolar plasma protein concentration may also affect these factors. These findings, predicted on theoretical grounds (
20), were confirmed using dextran under conditions of different glomerular plasma flow rates, one of the determinants of the GFR. Other evidence that glomerular hemodynamics may affect glomerular permeability is provided by the observation that angiotensin-converting enzyme (ACE) inhibition reduces proteinuria. Another structural feature that may affect water or macromolecular flux through the glomerulus is the SPS first demonstrated in three-dimensional reconstruction of the glomerulus (
41). This space is bounded by the podocyte cell body and the filtration barrier. Filtrate leaves the SPS and enters the urinary space through small exit pores between cell bodies, which produces a high-resistance space that may be controlled by the podocyte (
3,
41,
42).
A recent contribution from Hausmann et al. (
43) suggests the possibility that extracellular potential differences could be a determinant of glomerular filtration. They studied the
Necturus maculosus (common mudpuppy), which has sufficiently large glomeruli to undertake such a study, and found a potential difference of 0.09 mV at a perfusion pressure of 20 cm H
2O that could be eliminated by perfusion with a cation such as protamine. They explained this phenomenon as a streaming potential. This concept was further elucidated in an accompanying editorial in which a dilute ionic solution passes through a negatively charged filter, the positive ions will distribute in a uniform fashion (
44). However, application of flow under pressure will cause the positive ions to flow through the filter where some will stick to the nonplasma side. Some of the negative ions will be reflected resulting in a streaming potential difference. Another editorial in the same issue suggests caution in the interpretation of these results (
45).
Mechanisms of Proteinuria and the Effacement of Foot Processes
In general, proteinuria may result when there is addition of protein to tubular fluid (Tamm-Horsfall protein), altered tubular reabsorption, or altered glomerular permeability. We discuss only the last in this section and specifically address mechanisms that apply to those glomerular diseases that primarily have the NS as the initial manifestation.
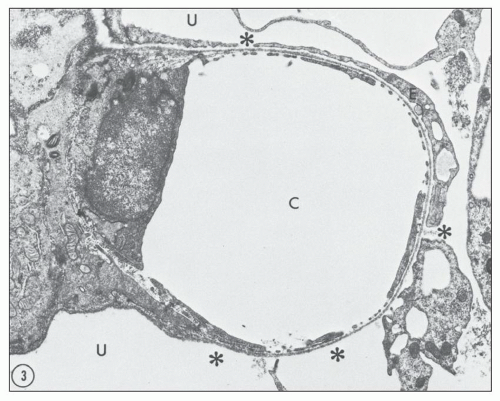
Numerous experimental models have been used to study possible mechanisms of proteinuria. The model of puromycin aminonucleoside nephrosis (PAN) has been used extensively over the years. This model shares many features of human MCD, and it is produced in rats by administering puromycin aminonucleoside (PA), either in a single injection or in several injections. A considerable proteinuria ensues that is due to increased glomerular permeability. Studies of the glomerular lesion using transmission and scanning electron microscopy (
47,
48) have revealed a replacement of foot processes by continuous sheets of flattened cytoplasm, epithelial vacuoles, a reduction in the number of epithelial slits with formation of occluding junctions, and focal areas where the epithelium has detached from the outside of the basement membranes (
Fig. 5.1). Other experimental models of proteinuric diseases as well as in human disease associated with proteinuria have similar changes.
The association between glomerular foot process effacement (FPE) and proteinuria is well known, but the mechanism of this effacement is complex and dynamic. In fact, podocyte motility is a term that is now used to reflect this dynamic view of the filtration barrier and changes in podocyte conformation (
49). Maintenance of foot processes may be deranged by alteration in negative charge of the apical domain of the podocyte, alterations in slit diaphragm, or interference with GBM-podocyte interaction (
50,
51). All three of these are functionally linked by the actin cytoskeleton (
52). The association between loss of negative charge from the podocyte and FPE has been known for some time. Seiler et al. (
53) showed that neutralization of glomerular polyanion by infusion of protamine, a polycation, produced the same flattening of epithelium, which was reversible by perfusion of polyanions. Loss of polyanion has also been demonstrated in PAN (
48) and following administration of anti-podoplanin antibody to rats (
54). Podoplanin is a mucin-like substance expressed on the surface of rat podocytes that contributes to the negative charge. Its removal by specific antibody results in massive proteinuria and FPE (
54). Additional evidence for a role for changes in the negative charge of the glomerular barrier was demonstrated by the recognition that angiopoietin-like protein 4 is up-regulated in the podocyte in MCD and is associated with both a loss of charge in the GBM and FPE mediated at least in part by a decrease in sialylation (
55,
56). This alteration is discussed in greater detail in the section on Pathogenesis of MCD.
The disruption of the components of the slit diaphragm in association with proteinuria and FPE has been demonstrated in CNS of the Finnish type (
57) as well as in knockout models for nephrin and CD2AP (
58,
59). FPE is always seen in these models accompanied by loss of the slit diaphragms. The proteins of the slit diaphragm are connected to the actin cytoskeleton by adapter proteins. Using the PAN model, Ito et al. (
60) demonstrated that activation of the mammalian target of rapamycin complex 1 preceded the occurrence of endoplasmic reticulum stress and the unfolded protein response that resulted in cytoplasmic location of nephrin and disruption of the filtration slit diaphragm.
Attachment of the foot processes to the GBM is mediated by laminin, dystroglycan, and α3β1-integrin (
61). Noakes et al. (
62) examined a mouse model with a null mutation for laminin-β2. The mice developed massive proteinuria associated with FPE and died by 1 month of age. Others have examined the role of abnormalities in dystroglycan in detachment of the podocyte from the GBM. The dystroglycan complex mediates adhesion at the basal cell membrane of the foot process by connecting matrix protein of the GBM and the actin network of
the podocyte. Vogtlander et al. (
63) noted that reactive oxygen species can cause deglycosylation of dystroglycan that may be associated with FPE. Reductions in dystroglycan have been seen in both MCD and focal segmental glomerulosclerosis (FSGS) (
61,
64).
As stated above, the three membrane domains on the podocyte are linked by the actin cytoskeleton, which has become a central focus in the concept of changing podocyte phenotype from stationary identified by the presence of intact foot processes to motile as associated with FPE. Prominent actin filaments are frequently seen in the effaced foot processes in human disease. Changes in the actin cytoskeleton have also been noted in PAN (
48) as well as in an autosomal dominant form of FSGS that has mutations in α-actinin (
65).
The regulation of the actin cytoskeleton is complex and is not yet completely understood. Nephrin in the slit diaphragm interacts with the actin cytoskeleton via various adapter proteins, notably CD2AP, Nck, and Crk (
52,
66). B7-1 (CD80) is a transmembrane molecule regularly found on B cells and antigen-presenting cells that may also be expressed on podocytes (
67). It is up-regulated by lipopolysaccharide (LPS), and such up-regulation was associated with actin reorganization in podocytes as well as a loss of slit diaphragms in vitro (
67). Cathepsin L-mediated proteolysis particularly of dynamin and synaptopodin, important in maintaining podocyte stability, has been demonstrated to play a critical role in changing podocyte phenotype to motility (
52,
68). Activation of RhoA, a member of the family of small GTPases, is associated with actin polymerization, but its inhibition also resulted in actin polymerization, suggesting that RhoA must be tightly regulated (
49,
69).
Several investigators have found that the onset of heavy proteinuria coincides with epithelial detachment (
70). Furthermore, ultrastructural tracer techniques have shown penetration of anionic ferritin into the urinary space at these detachment sites (
48). Use of the technique of multiphoton fluorescence imaging in vivo in the PAN model confirmed areas of increased glomerular permeability near damaged podocytes as well as real-time shedding of podocytes (
37). FPE is a reversible change, but if it progresses to foot process detachment, then irreversible injury may result (
52).
Effects of Proteinuria on the Tubules and Interstitium
For many years, the question of damaging effects of proteinuria on the tubules and interstitium has been raised. The fact that patients with steroid-dependent MCD could suffer from nephrotic-range proteinuria for years and not show such injury spoke against the idea. However, mounting evidence suggests that at least nonselective proteinuria may result in such injury. Albumin, various vitamins, and other substances in tubular fluid are normally nearly completely reabsorbed by the tubular epithelium by receptor-mediated endocytosis (
71). Uptake of albumin requires cubilin with endocytosis of the albumin-cubilin complex and transport to the lysosome necessitating the presence of megalin (
72). It has been proposed that the various substances may directly cause tubular toxicity, that growth factors and other substances may cause up-regulation of cytokines/chemokines, or that complement may be activated (
73,
74,
75,
76). Theilig et al. (
77) induced crescentic glomerulonephritis (GN) in transgenic megalindeficient mice. The lack of expression of megalin was mosaic so that megalin-deficient tubules could be compared to those that expressed megalin. This comparison showed endocytosis and an up-regulation of TGFβ in the megalin-positive cells, while those deficient in megalin demonstrated apoptosis. However, neither group of tubules showed surrounding interstitial fibrosis. Kriz (
77,
78) believes that this experiment supports his contention that it is severe glomerular damage that is associated with downstream tubulointerstitial injury rather than tubular reabsorption of leaked proteins. Further experiments are necessary to dissect these possibilities.