Renal Injury Associated With Human Immunodeficiency Virus Infection and Therapy
Arthur H. Cohen
Cynthia C. Nast
It was within 3 years of the initial description of the acquired immunodeficiency syndrome (AIDS) that the first reports of renal involvement appeared (1,2,3) and documented various forms of glomerular disease. Since that time, myriad publications have provided ample evidence that the kidneys may and do harbor varied serious and pathogenically diverse lesions and manifest numerous clinical syndromes in human immunodeficiency virus (HIV)-infected patients (4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21). It has become apparent that some of the lesions may be directly related to HIV infection of the kidney, some may be the result of immunologic responses to the virus, some may be indirect manifestations of viral infection not within the kidneys, still others evolve from the opportunistic infections and neoplasms that characterize AIDS, and others may be the consequences of various therapies or hemodynamic derangements. It has also become apparent that a few types of nephropathy may occur in asymptomatic HIV-infected individuals and that the more serious manifestations of HIV infection are not always necessarily present in patients with renal disease.
In this chapter, we consider in detail those lesions that are unique to (or virtually limited to) HIV infection and those lesions that are associated with various reasonably consistent diseases common to HIV infection and AIDS or their complications, and we discuss in less detail lesions that are seen in patients with AIDS but that are covered more comprehensively in other chapters. It has been estimated that ≤60% of patients (10,22) with AIDS will manifest clinically significant kidney dysfunction, whether acid-base or electrolyte disturbances or parenchymal damage. Based upon racial and geographic considerations, the nature of the lesions varies considerably. It is important to note, however, that, as documented by autopsy studies (23,24), different statistics with regard to renal abnormalities
emerge. That is, postmortem examinations of consecutive and unselected patients have indicated that many of the lesions encountered in renal biopsy series are rarely observed in autopsies. That information has shed some doubt on the clinically derived statistics, although it may well be that our clinical colleagues’ experience is more accurate. As of 1999, HIV-related renal disease was the third leading cause of end-stage renal disease among African Americans 20 to 64 years of age. However, due to effective treatment, this risk has declined by 40% to 60% accompanied by a threefold increased survival on dialysis (25). In addition, some investigators have suggested that, while HIV-associated nephropathy (HIVAN) was once considered the major renal lesion in HIV infection, it is likely that all other pathologies are collectively almost as or more common in the era of highly active antiretroviral therapy (HAART) (26).
emerge. That is, postmortem examinations of consecutive and unselected patients have indicated that many of the lesions encountered in renal biopsy series are rarely observed in autopsies. That information has shed some doubt on the clinically derived statistics, although it may well be that our clinical colleagues’ experience is more accurate. As of 1999, HIV-related renal disease was the third leading cause of end-stage renal disease among African Americans 20 to 64 years of age. However, due to effective treatment, this risk has declined by 40% to 60% accompanied by a threefold increased survival on dialysis (25). In addition, some investigators have suggested that, while HIV-associated nephropathy (HIVAN) was once considered the major renal lesion in HIV infection, it is likely that all other pathologies are collectively almost as or more common in the era of highly active antiretroviral therapy (HAART) (26).
HIV-ASSOCIATED NEPHROPATHY
The seminal report in March 1984 by Rao et al. in Brooklyn, New York (3), of focal segmental glomerulosclerosis in a group of black intravenous heroin abusers with AIDS, represented the first formal awareness that some patients with AIDS may show signs of renal disease. Before the end of that year, Pardo et al. in Miami (2) and Gardenswartz et al. in New York City (1) also reported clinically significant parenchymal renal disease, especially glomerular lesions, in patients with AIDS. In all three reports, the major glomerulopathy was described as focal and segmental glomerulosclerosis. Over the course of the next 5 or 6 years, detailed aspects of the clinical manifestations and epidemiologic considerations were made known, and comprehensive pathologic descriptions were written and refined (5,27,28,29,30,31,32,33,34,35,36,37). Since then, considerable attention has been directed to elucidating pathogenetic aspects of the disorder now known as HIV-associated nephropathy (HIVAN). The condition has reasonably characteristic clinical manifestations, striking racial predominance, and exciting and intriguing pathologic features.
HIVAN is a pan-nephropathy, with regularly occurring glomerular, tubular, and interstitial changes. As is described in detail later, the glomerular lesion is the collapsing variant of focal segmental glomerulosclerosis; the tubular abnormalities include cellular degeneration and necrosis, as well as cystic dilation, and the interstitium is edematous and often infiltrated by lymphocytes. Thus, while many other glomerulopathies and parenchymal lesions have been described in HIV-infected patients, it is only the one discussed in this section that should be termed HIVAN, based upon proposed pathogenetic mechanisms and historical considerations (29,38). All other lesions should be known by their commonly accepted appellations, with further indication of their existence in an HIV-infected individual. It makes no sense to consider all renal lesions in patients with HIV as HIVAN (37,38); certainly, we do not designate all forms of renal injury in patients with diabetes mellitus as diabetic nephropathy.
Clinical Features
Although the use of HAART appears to have an impact on the presentation and course of HIVAN (26), this description will pertain to the untreated patient. The effects of therapy are described below. HIVAN typically appears with the acute onset of heavy proteinuria (often ≥20 g/d) and progressive renal insufficiency. Hypoalbuminemia is usual, and proteinuria is nonselective. Renal failure may occur acutely, and some patients may have mild renal insufficiency. In contrast to other nephropathies with massive proteinuria, there is no or little peripheral edema, and blood pressure is often normal, even with advanced renal insufficiency (4,9,10,22,37,39). Most patients have had HIV infection for several years and have low CD4 counts. In the HAART era, more patients present earlier and with higher CD4 counts. The progression to end-stage renal disease may be quite rapid; in some series, the time from onset to dialysis may be as little as a few weeks to a few months (27,33,37). This progression is often irrespective of the clinical manifestations of HIV infection. It appears that black patients have a more severe course than either Hispanic or non-Hispanic white patients (40,41,42).
Imaging studies have consistently noted normal-sized or, more commonly, enlarged kidneys not only initially but also during the course of the disease, including end-stage kidney disease (9,43). Nephromegaly is so characteristic that an astute clinician can strongly suspect HIVAN on the basis of the clinical and laboratory features in conjunction with enlarged kidneys.
Although the initial reports documented this entity in patients with full-blown AIDS, with the advent of serologic testing for HIV, it has become evident that this nephropathy can manifest at any stage of HIV infection (26,28,29,30,43,44). In patients who are otherwise asymptomatic for HIV infection, this nephropathy may be the initial manifestation, and the renal pathologist may be the first physician to diagnose HIV infection based upon the biopsy findings (28,29,30,45,46). At the present time, although it is a common clinical practice to include HIV testing in the work-up of patients with renal disease, renal biopsy continues to represent the diagnostic method of detecting HIV infection in a small number of patients.
Patients with HIVAN acquire HIV infection by any of the modes of transmission (29,30). It was initially thought that intravenous drug abuse was an important and even necessary cofactor (3,11,17,43). However, many reports have documented the existence of this nephropathy in children with perinatal transmission of the virus, adults with heterosexual transmission, male homosexuals without intravenous drug abuse, and patients with HIV infection from contaminated therapeutic blood products (8,9,19,29,47).
From the first reports, it has been abundantly clear that there is a striking racial predominance—80% to 85% of reported patients are black. First pointed out by Bourgoignie et al. (48) in 1989 in a review of the literature, this racial imbalance has been amply supported by all published series (49). Bourgoignie et al. (39) convincingly documented their own experience; in 1989, in Jackson Memorial Medical Center in Miami, 67% of HIV-infected patients were white (both Hispanic and non-Hispanic), yet 88% of HIVAN patients were black. The incidence and prevalence of this lesion in Africa are unknown, although it clearly occurs in native Africans; several reports from Europe have described this nephropathy in Africans and residents of the Caribbean islands who seek treatment at European medical centers (15,50). Reports from Africa indicate the emergence of HIVAN and other renal diseases in patients with longer duration of illness, perhaps reflecting the effect of anti-HIV therapy (31,51,52,53). It is of interest to note that Pakasa et al. (54) described an increasing incidence of focal segmental glomerulosclerosis, including the collapsing variant (see Chapter 6) in Kinshasa, Zaire, although specific information about HIV status was not known in most patients. Few reports have documented rare cases in Hong Kong and India, among a small number of Asian countries describing their experience (55,56).
The racial predominance also explains the geographic and other seemingly anomalous aspects of epidemiology that have become evident. For example, for the first several years following the awareness that some AIDS patients had coexisting focal segmental glomerulosclerosis, reports denying the existence of “AIDS-associated” glomerulopathy appeared in the medical literature in both the United States and Europe (57). However, it soon became obvious that the medical centers with this view treated very few or no black patients; with experience with a more racially heterogeneous patient population, some doubters became believers and proponents (31)
Pathology
Gross
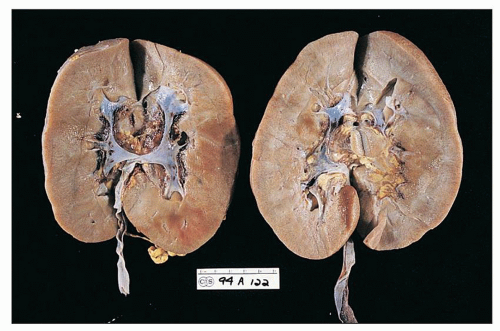
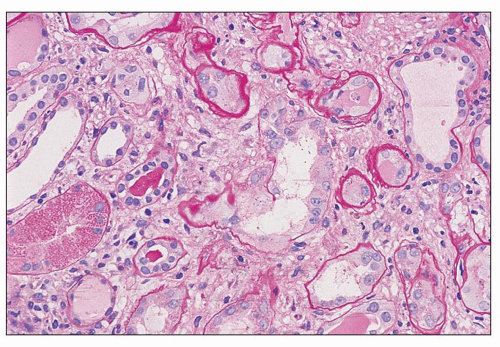
The kidneys are generally enlarged, even at end stage (Fig. 11.1). In adults, the mean combined kidney weight has been documented to be as high as 500 g (58). In children, the mean weight is usually 1.20 to 1.30 times normal (22). The capsular surface is smooth. The cortex is enlarged and is more pale than the medulla. The parenchyma is swollen. Small cysts, 0.5 to 1.0 mm, are occasionally observed in the cortex or at the corticomedullary junction; they correspond to massively dilated tubules. As a rule, unless hypertension or chronic renal disease existed before the onset of HIVAN, grossly discerned scarring is not a feature.
Light Microscopy
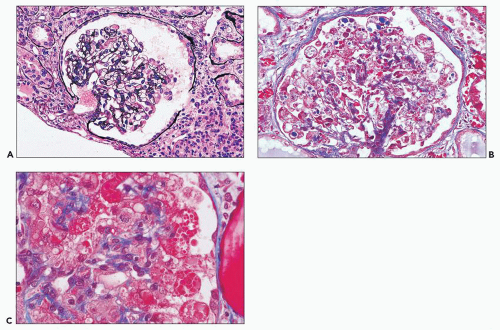
There are prominent coexisting changes of glomeruli and tubules, often with an important interstitial component. The lesions of glomeruli are most typically, but not always, of the collapsing variant of focal segmental glomerulosclerosis (59). Because HIVAN has both clinical and pathologic manifestations of tubulointerstitial as well as glomerular involvement, the designation “nephropathy” rather than focal and segmental glomerulosclerosis is appropriate. As we view the glomerular abnormalities, they represent predominantly the early lesions of focal segmental glomerulosclerosis (“shift to the left” in the morphogenesis) (29,38,60). Initially, there are hypertrophy and hyperplasia of visceral epithelial cells; these alterations may affect only a few cells of a single lobule (Fig. 11.2A) or virtually all epithelial cells in a glomerulus simulating a crescent (Fig. 11.2B). Parietal epithelial cells have
been shown to contribute to the glomerular epithelial cell hyperplasia in collapsing glomerulopathy (61). Mitotic figures are usually present in some epithelial cells. The abnormal cells may be coarsely vacuolated and characteristically contain many cytoplasmic protein reabsorption droplets (Fig. 11.2C). Capillary walls in affected segments may be wrinkled or collapsed and lumina narrowed. There is relative dilation of the urinary space with segmental or more extensive involvement. As the lesion evolves, there is further narrowing or obliteration of lumina, resulting in solidification of the tufts (Fig. 11.3). In some glomeruli, typical segments of sclerosis develop, with lumina containing foam cells, insudative lesions, or both (Fig. 11.4). In advanced lesions, the visceral epithelial cells remain numerous and enlarged with protein resorption droplets and cytoplasmic vacuoles; the cells are typically arranged as a crown over the solidified tuft or a portion thereof (Fig. 11.5) (5,9,22,29,30,38,58).
been shown to contribute to the glomerular epithelial cell hyperplasia in collapsing glomerulopathy (61). Mitotic figures are usually present in some epithelial cells. The abnormal cells may be coarsely vacuolated and characteristically contain many cytoplasmic protein reabsorption droplets (Fig. 11.2C). Capillary walls in affected segments may be wrinkled or collapsed and lumina narrowed. There is relative dilation of the urinary space with segmental or more extensive involvement. As the lesion evolves, there is further narrowing or obliteration of lumina, resulting in solidification of the tufts (Fig. 11.3). In some glomeruli, typical segments of sclerosis develop, with lumina containing foam cells, insudative lesions, or both (Fig. 11.4). In advanced lesions, the visceral epithelial cells remain numerous and enlarged with protein resorption droplets and cytoplasmic vacuoles; the cells are typically arranged as a crown over the solidified tuft or a portion thereof (Fig. 11.5) (5,9,22,29,30,38,58).
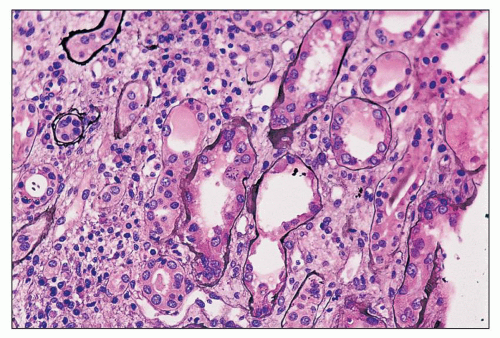
There are constant and varied abnormalities of the tubules. Cells display degenerative features, with diminished or absent brush border staining of proximal tubular cells, flattening of cells in many segments of the nephron, and sloughing of cells into the lumina (Fig. 11.6). These changes may be focal or, more commonly, widespread (29,30,38). Lumina of tubules, in all segments of the nephron, are characteristically filled with a precipitate of plasma protein (Fig. 11.7), staining pale or negative with periodic acid-Schiff (PAS) and fuchsin positive with Masson trichrome. Tubular lumina are dilated, some only modestly and others massively, forming microcysts that may be visible on gross examination. Some tubules are so markedly dilated that they dwarf adjacent glomeruli (Fig. 11.8). Bowman spaces, also dilated and in direct continuity with dilated proximal tubules, contain the same precipitate (Fig. 11.9). The luminal precipitates, whether in greatly or slightly dilated tubules, have a scalloped periphery at the interface with apical portions of epithelial cells. Some of the luminal precipitates may contain more densely staining casts, crystals, or desquamated cells or cellular debris. In addition, some nondilated tubules contain typical hyaline casts composed of Tamm-Horsfall protein; as expected, these are strongly PAS positive. Other changes of tubular epithelium include the accumulation of numerous protein reabsorption droplets in proximal cells (Fig. 11.10) (29,38).
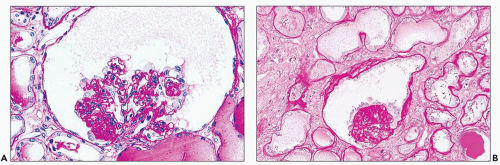
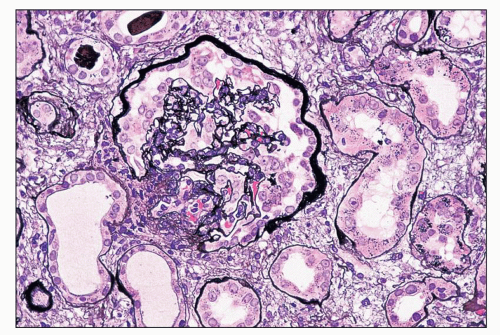 FIGURE 11.3 A glomerulus shows more advanced changes than in Figure 11.2. Portions of tufts are solidified because of capillary wall collapse. The epithelial cells are diffusely hyperplastic and exhibit changes similar to those in the earlier figures. (Periodic acid-methenamine silver, ×80.) |
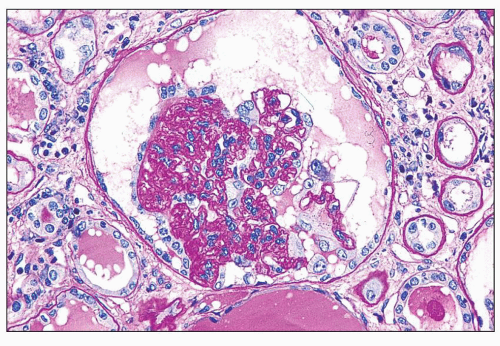 FIGURE 11.4 Glomerulus with advanced changes including segmental collapse and solidification of the tufts with a capsular adhesion and few enlarged podocytes. (PAS, ×80.) |
The interstitium is edematous and contains a variable infiltrate of lymphocytes, with fewer plasma cells and monocytes (29,30,38). Interstitial fibrosis and tubular atrophy are present as the disease progresses; alternatively, these features may be related to other chronic changes (e.g., hypertension and nephrosclerosis) antedating the development of HIVAN. There are no consistent accompanying alterations of arteries, arterioles, or veins. Hypertension as an integral feature of HIVAN is unusual. Vascular changes of nephrosclerosis are most commonly associated with preexisting hypertension.
Immunofluorescence and Immunohistochemistry
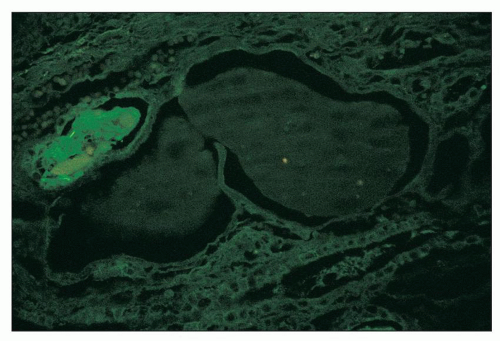
The immunofluorescence findings in glomeruli are as would be expected for focal and segmental glomerulosclerosis, including the collapsing variant. In glomeruli with segmental sclerosis, immunoglobulin M (IgM), C3, and C1q are typically found in capillary walls in a coarsely granular to amorphous pattern with a segmental distribution, corresponding in location to the abnormal segments (27,28,36). “Noninvolved” glomeruli usually show negative results on immunofluorescence, although IgM and C3 have been documented in mesangial regions in a granular pattern and with a diffuse distribution in some reports. Most commonly, albumin, but also IgA, IgG, or both, is present in visceral epithelial cells as protein resorption droplets (29,30,38) (Fig. 11.11). Similarly, albumin, with lesser amounts of IgG, IgA, complement, and both light chains, is found in proximal tubular cells as protein resorption droplets. In our study of the composition of the precipitates (“casts”) in dilated tubular lumina, we documented the presence of all plasma proteins sought (IgG, IgA, IgM, C1q, C3, albumin, both light chains) but not Tamm-Horsfall protein
(Fig. 11.12), strongly suggesting their origin to be of the glomerular filtrate. In contrast, typical tubular hyaline casts are composed of Tamm-Horsfall protein, IgA, variable IgM, and both light chains, findings typical for these casts in other settings (62).
(Fig. 11.12), strongly suggesting their origin to be of the glomerular filtrate. In contrast, typical tubular hyaline casts are composed of Tamm-Horsfall protein, IgA, variable IgM, and both light chains, findings typical for these casts in other settings (62).
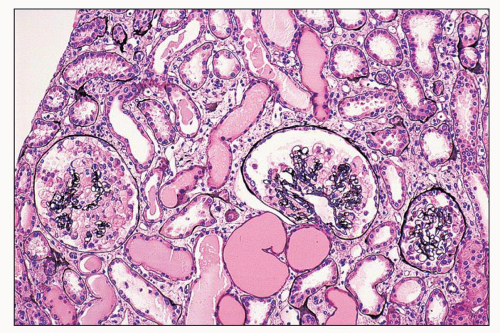 FIGURE 11.7 Many tubules are filled with a pale-staining luminal precipitate; some are also slightly dilated. Note glomerular changes. (Periodic acid-methenamine silver, ×40.) |
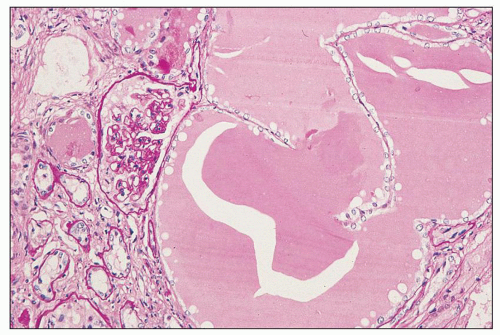 FIGURE 11.8 Massive dilation of tubules filled with plasma protein. This is from the autopsy of the patient whose biopsy is depicted in Figure 11.7; he died 3 months after the biopsy. (PAS, ×16.) |
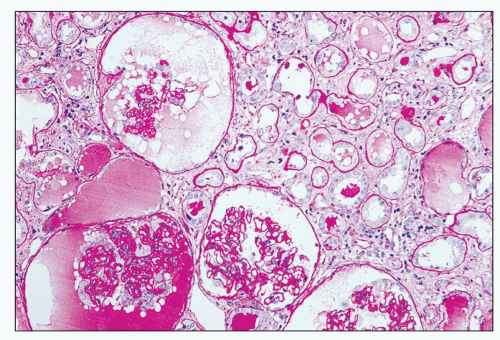 FIGURE 11.9 Tubules with moderate luminal dilation and proteinaceous precipitates. Note the Bowman spaces of glomeruli are similarly affected. (PAS, ×40.) |
Cell marker studies have elucidated the composition of the leukocytic interstitial infiltrate. In the study by D’Agati et al. (30), there were both CD4 and CD8 cells, with a markedly reduced CD4/CD8 ratio (0.23 to 0.77, mean 0.35), similar to findings in the peripheral blood. In contrast, Rey et al. (63), studying mostly patients with full-blown AIDS, identified the infiltrating cells to be predominantly T cells, with both CD4 and CD8 cells present in a ratio of approximately 1.0, considerably different from the situation with circulating blood, which had markedly diminished numbers of CD4 cells and more numerous CD8 cells. These authors documented expression of major histocompatibility complex class II molecules (HLA-DR) on these cells as well as on parenchymal cells (endothelial, glomerular mesangial, and visceral epithelial cells). T cells were rarely identified in tubules or glomeruli. B cells and macrophages represented a minor component of the infiltrate. This study indicates that while AIDS may be associated with considerable CD4 lymphopenia, some organs may harbor a disproportionate number of these cells. Bodi et al. (64) documented that approximately 50% of interstitial leukocytes were macrophages, approximately 3% were B cells, and the remainder were T cells; it is not clear from their report what manifestations of HIV infection the patient population exhibited, making comparison with the other two studies difficult. It is of interest to note that Rey et al. (60,63) found the infiltrate in HIVAN to differ from that of idiopathic focal segmental glomerulosclerosis, which is characterized by considerably higher CD4/CD8 ratios.
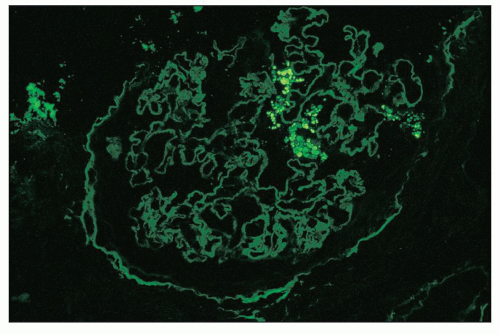 FIGURE 11.11 Many glomerular podocytes contain protein resorption droplets stained for albumin. (×80.) |
Electron Microscopy
The ultrastructural findings reflect two distinctive processes and features: the basic electron microscopic features of the glomeruli and tubulointerstitium in focal segmental glomerulosclerosis/collapsing glomerulopathy (65,66,67) and the fine structural abnormalities of HIV infection (28,30,38,45,62,68,69). Each is discussed separately.
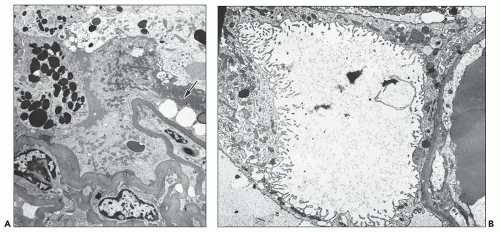
In the case of glomerulopathy, the abnormalities are dominated by pronounced changes in visceral epithelium and mirror very well the features of light microscopy (27,28,36). In affected glomeruli or segments thereof, these cells are often massively enlarged and contain one or more dense, round, secondary lysosomes (Fig. 11.13A); in addition, large and irregular single membrane-bound and seemingly empty vacuoles are present (see Fig. 11.13B). The vacuoles are often responsible for bizarre and irregular shapes assumed by these cells. The vacuoles may be exceedingly large, larger than the caliber of the underlying capillary lumen. The foot processes are usually completely effaced. The epithelial cell cytoplasm is not infrequently partially or completely detached from the basement membrane (Fig. 11.14A); at a later stage, after repair, the cell is separated from the original basement membrane by thin layers of presumably new basement membrane material (Fig. 11.14B).
Basement membranes display varying degrees of wrinkling with luminal narrowing (29,38). Endothelial cells may be swollen. With advancing lesions, capillary lumina may be completely obliterated by basement membrane material. Less commonly, monocytes with considerable cytoplasmic lipid (foam cells) fill the lumina, often in association with degeneration and detachment of endothelial cells. With further progression, plasma protein insudates, in the form of extracellular electron-dense masses, often with incorporated lipid and debris, occlude the lumina (38). Electron-dense deposits are usually absent, although small deposits in the mesangium are present in glomeruli with mesangial IgM and C3 deposits.
There are several ultrastructural abnormalities associated with HIV infection; it should be recognized that they are not limited to the kidneys but are widespread in many organs (68,69,70). Thus, their presence does not define the kidney changes as being indicative of HIVAN but as occurring in an HIV-infected patient. That is, there is a prevalent misconception that when these ultrastructural abnormalities are present in the kidneys, they are indicative only of HIVAN or a lesion
directly related to HIV infection. However, they are indicative only of HIV infection and are present in the kidneys with other lesions, ranging from IgA nephropathy perhaps integrally related to HIV to, for example, diabetic nephropathy in an HIV-infected patient.
directly related to HIV infection. However, they are indicative only of HIV infection and are present in the kidneys with other lesions, ranging from IgA nephropathy perhaps integrally related to HIV to, for example, diabetic nephropathy in an HIV-infected patient.
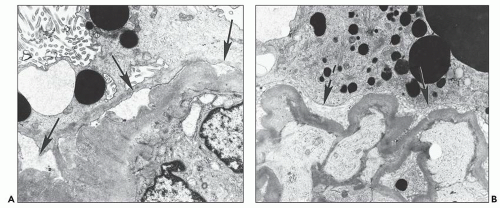
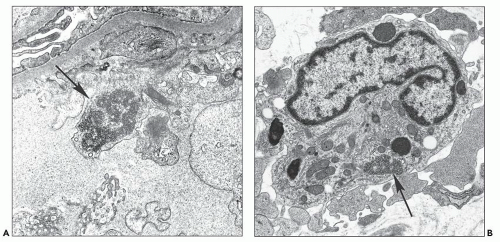
The most common abnormality is the tubuloreticular structure (Fig. 11.15A), consisting of reticular aggregates of branching tubules, approximately 25 nm in diameter, within cisternae of the endoplasmic reticulum or the nuclear envelope (68,69). These cytoplasmic structures, also known as tubuloreticular inclusions or interferon footprints, are primarily found in endothelial cells of glomeruli and peritubular capillaries and, to a lesser degree, in arteries, arterioles, and veins; in addition, they may be present in lymphocytes and monocytes (Fig. 11.15B), whether in the interstitium or in vessel lumina. They are typically large and numerous. Tubuloreticular structures are considered to be induced by cellular exposure to interferon-alpha. They are also an integral feature of cells in patients with systemic lupus erythematosus and in patients who are treated with interferon-alpha and may be found in patients with other viral infections (71). In addition, tubuloreticular structures may be identified, although in small numbers, in other forms of renal disease. Therefore, these structures are not specific to HIV infection.
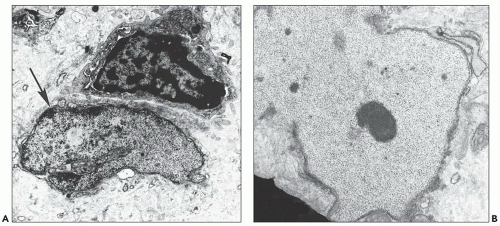
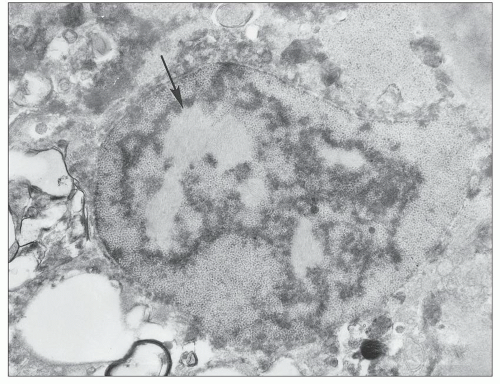
Another ultrastructurally defined lesion, cylindrical confronting cisternae, is emphasized as an important and regularly occurring abnormality (28,69), but it is infrequently noted in renal tissue. These cisternae are long cylinders of fused membranous lamellae also known as test tube- and ring-shaped forms (Fig. 11.16); they almost always occur in cells with tubuloreticular structures, mainly in lymphocytes and monocytes. Confronting cylindrical cisternae are not unique to HIV infection; they also have been documented in multiple sclerosis, systemic lupus erythematosus, and in association with interferon-alpha therapy. Nuclear alterations are not uncommon. Perhaps, the most typical are nuclear bodies; these are nuclear inclusions, with diverse morphologic characteristics, consisting of dense or pale granular or fibrillary aggregates (Fig. 11.17). They are usually divided into five different types based upon structure (68) and are found most often in tubular and interstitial cells, including leukocytes and fibroblasts, where they are frequently quite numerous in a single nucleus.
The type III variant is most commonly present (30,38). Nuclear bodies are not specific to HIV infection; they had been described many years before the onset of the AIDS epidemic. Their composition and function are unknown; they may occur in association with viral infections. At the present time, this group of structures no longer generates the same interest as earlier.
The type III variant is most commonly present (30,38). Nuclear bodies are not specific to HIV infection; they had been described many years before the onset of the AIDS epidemic. Their composition and function are unknown; they may occur in association with viral infections. At the present time, this group of structures no longer generates the same interest as earlier.
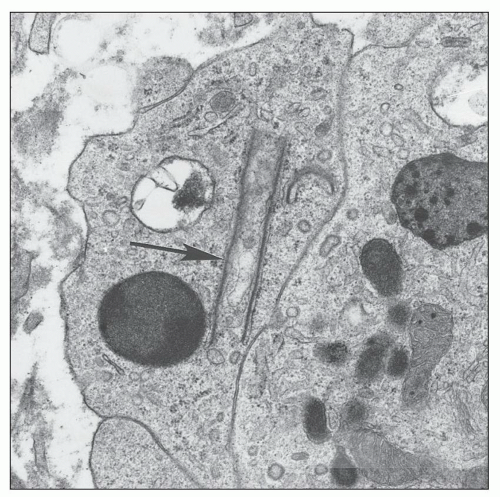 FIGURE 11.16 Confronting cylindrical cisternae (arrow) in the cytoplasm of an interstitial monocyte. (×19,450.) |
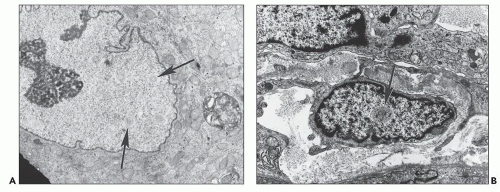 FIGURE 11.17 Nuclear bodies. A: A tubular epithelial cell with six type I nuclear bodies (arrows point to two). (×6800.) B: An interstitial cell with a single type III nuclear body (arrow). (×9980.) |
More pronounced nuclear changes also may be present, including granular transformation of nuclei and, less commonly, granulofibrillar transformation. In the former case, the nucleus is completely or partially replaced by a coarsely granular material often associated with disruption of a portion of or the complete nuclear membrane (Fig. 11.18). Concomitant alterations of cytoplasm and organelles, especially mitochondrial swelling, are not uncommon. It should be noted that this change is not an artifact of inadequate fixation, for nuclei and cytoplasm of adjacent cells are not similarly abnormal. This alteration typically affects tubular and interstitial cells (28,36); it is more frequently found in interstitial cells in our experience (38).
Another more dramatic and perhaps related lesion is granulofibrillar transformation of nuclei; the chromatin is replaced by material with a coarse or fine granularity, with interspersed bundles of pale-staining filamentous (“fibrillar”) structures
(Fig. 11.19). While granular transformation is readily seen in biopsy material, it should be noted that granulofibrillar transformation seems to be restricted to postmortem specimens (38,68,69). The nature of the transforming material is not known in either instance. The specificity of granular transformation to HIV infection is not known; we have noted it occasionally in renal biopsies from patients with systemic lupus erythematosus, among other disorders. While cytomegalovirus (CMV) and other viruses have been identified in renal tissue in HIVAN and represent coexisting disease rather than having pathogenetic importance in this disorder, the HIV agent has not yet been described in any renal cell.
(Fig. 11.19). While granular transformation is readily seen in biopsy material, it should be noted that granulofibrillar transformation seems to be restricted to postmortem specimens (38,68,69). The nature of the transforming material is not known in either instance. The specificity of granular transformation to HIV infection is not known; we have noted it occasionally in renal biopsies from patients with systemic lupus erythematosus, among other disorders. While cytomegalovirus (CMV) and other viruses have been identified in renal tissue in HIVAN and represent coexisting disease rather than having pathogenetic importance in this disorder, the HIV agent has not yet been described in any renal cell.
The tubular casts of plasma protein are of medium density, with a finely granular to homogeneous appearance, without fibrils typical of Tamm-Horsfall protein (62), and without cytoplasmic or other debris. The casts look the same, regardless of the nephron segment in which they are located (29). Morphologic tubular cell manifestations reflecting acute tubular necrosis and tubular reabsorption of protein are similar to those seen in other nephropathies. The interstitial changes, likewise, are not different from those found in other disease contexts.
The light microscopic features, especially of the collapsing form of focal segmental glomerulosclerosis with tubular cell changes, tubular luminal protein precipitates, and cysti-cally dilated tubules—in conjunction with the ultrastructural findings (particularly, but not exclusively, numerous, large, and widespread tubuloreticular structures)—are a fairly unique combination of findings. Observed together, these are highly suggestive of HIVAN, and HIV infection should be confirmed by testing for HIV with polymerase chain reaction (PCR) or for HIV antibodies serologically. The differential diagnosis includes patients treated with interferon, which can induce a picture of collapsing glomerulopathy with tubuloreticular structures (72). This constellation of findings allows the renal pathologist to suggest a diagnosis of HIV infection in a patient not previously known to harbor the virus (27,28,29,44,45,46). It should be emphasized that the diagnostic criteria must include electron microscopic evaluation as collapsing glomerulopathy clearly occurs in non-HIV-infected persons, some of whom may have other immunodeficiency conditions; if light microscopy alone is used, diagnostic specificity is lost. Thus, incompletely studied renal specimens are likely to provide misleading information.
In the HAART era, tubuloreticular structures are much less commonly found (21,73). Some specimens may be devoid of them, and fewer tubuloreticular structures are present in those biopsies that harbor them. Although the consequence of
these features is that they have a reduced role in pathologic diagnostic specificity, the pathologist is very likely to be aware that the patient is HIV infected at the time of the renal biopsy. Additionally, since the introduction of HAART, the prevalence of the typical morphologic changes of HIVAN has decreased, while the “classical” (non-collapsing) forms of focal segmental glomerulosclerosis have increased likely due to effectiveness of HAART in abrogating the collapsing glomerulopathy of HIVAN (74,75). Over the last several years, the noncollapsing morphology is the leading cause of glomerular disease in HIV-infected individuals followed in one large institution in Paris (26).
these features is that they have a reduced role in pathologic diagnostic specificity, the pathologist is very likely to be aware that the patient is HIV infected at the time of the renal biopsy. Additionally, since the introduction of HAART, the prevalence of the typical morphologic changes of HIVAN has decreased, while the “classical” (non-collapsing) forms of focal segmental glomerulosclerosis have increased likely due to effectiveness of HAART in abrogating the collapsing glomerulopathy of HIVAN (74,75). Over the last several years, the noncollapsing morphology is the leading cause of glomerular disease in HIV-infected individuals followed in one large institution in Paris (26).
Natural History
Untreated, this nephropathy has a uniformly dismal prognosis, with or without clinical manifestations of AIDS. In 1989, Carbone et al. (27) described rapid progression to renal insufficiency in all patients (HIV asymptomatic, AIDS-related complex, AIDS), from the initial clinical presentation to dialysis in 10.9 weeks. The median survival time from diagnosis of renal disease to death was 4.5 months. In asymptomatic patients, it was 9.7 months; in those with AIDS-related complex, it was 3 to 6 months; and in those with AIDS, it was 1.9 months. This rate was not appreciably different from the initial experience of Rao et al. 5 years earlier (3). Some reports have indicated a better outcome in patients treated with zidovudine (35,76,77) although this is not uniformly true (78).
Examination of renal tissue some time after the initial pathologic diagnosis of HIVAN, either with a second biopsy or at autopsy examination, indicates progression of the glomerular and tubulointerstitial damage. We have noted marked solidification of collapsed tufts, commonly with persistent enlargement and vacuolation of visceral epithelial cells of the vast majority of glomeruli at autopsy, in as little as 3 months following a biopsy that disclosed exclusively glomerular epithelial hypertrophy and hyperplasia with early capillary wall collapse in a patient who experienced rapid decline to end-stage renal disease. Concomitant massive tubular dilation, not present on biopsy, was also evident in the postmortem specimen. Few studies have assessed morphologic progression in the face of corticosteroid therapy. Briggs et al. (79) reported the case of a patient with combined typical HIVAN and thrombotic microangiopathy who was treated with prednisone (60 mg/d) and experienced initial improvement in renal function; following tapering of prednisone, renal function deteriorated. A second biopsy showed “substantial” reduction of interstitial inflammation, with fewer T cells and macrophages/monocytes. The glomerulopathy was unchanged (as was proteinuria), and vascular thrombotic microangiopathic lesions were absent.
Since the introduction of HAART, data indicate that, as HIV-infected patients are living longer, the incidence of kidney involvement with progression to end-stage renal disease is increasing. Some treatment modalities have an effect on the incidence and progression of HIVAN. Patients with HIVAN treated with angiotensin-converting enzyme inhibition show delayed progression to end-stage renal disease. Antiretroviral therapy has been reported to result in dramatic improvement in HIVAN and longer time to renal failure (80), and HIVAN is considered an indication for HAART administration (81). Furthermore, few isolated case reports (82) have described considerable improvement in renal function and proteinuria as well as microscopic pathology in some patients with biopsy-documented HIVAN (83,84).
Etiology and Pathogenesis
Since its initial description, the pathogenesis of HIVAN has been a source of considerable interest and investigation. Although many diverse theories have been proposed, it seems very likely that HIV genome or protein in the renal epithelium is responsible for the development of this nephropathy (13,20,21,85). In an attempt to explain the simultaneous occurrence of the striking abnormalities in glomerular visceral and tubular epithelium and their functional consequences as manifestations of a single renal injury, we studied renal biopsy specimens for HIV nucleic acid with in situ hybridization using a cDNA probe and with a peroxidase-labeled antibody to p24 core protein (86). HIV genome was documented in many glomerular visceral and tubular epithelial cells in HIVAN; in contrast, in kidneys from HIV-infected patients with immune complex glomerulonephritis and in AIDS patients without renal disease, genome was documented in only isolated glomerular and tubular epithelial cells in very few tissue samples. The immunohistochemical part of the study indicated that this method was not very sensitive, for in only 1 of 11 biopsies with HIVAN was p24 identified in glomerular epithelium; tubular cells were positive in 10 of 11. These results suggested a role of direct HIV infection of these cells in the initiation, progression, or both of HIVAN. Originally discounted because other studies (87,88,89) could not reproduce the results in different settings, evidence over the last decade, using more sophisticated techniques, has confirmed the basic concept that HIV genome is an important, indeed necessary, factor in renal epithelium in the genesis of this disorder (83,90,91,92,93).
The methodologic problems associated with the immunohistochemical detection of HIV antigen in tissue sections were reviewed extensively by Nadasdy et al. (94); these investigators carefully documented the pitfalls of identifying viral protein in fixed and paraffin-embedded kidney and other tissue specimens. Kimmel et al. (95) documented HIV DNA by PCR in microdissected kidney biopsies; viral genome was identified in glomerular and tubular cells in HIVAN and also in other glomerulopathies in HIV-infected patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree