Renal Disease in Pregnancy
Agnes B. Fogo
EFFECTS OF NORMAL PREGNANCY ON THE KIDNEY
Structural Changes in Pregnancy
Pregnancy normally induces changes in kidney function. In some women, complications of pregnancy may cause renal disease, and even normal pregnancy can exacerbate preexisting renal disease. Kidneys increase in volume, as much as 70% by ultrasound studies, during pregnancy, largely secondary to increased fluid content (1). The calyces and ureters dilate markedly during pregnancy, beginning as early as the 7th week and progressing gradually until term. By 1 week postpartum, these portions of the collecting system return to the prepregnant state in one third of women. In an additional one third, this dilation reverts by 1 month postpartum, and nearly all remaining patients return to normal by 2 months postpartum (2). The dilation is nearly always more prominent on the right, possibly because of the abrupt angle of the right ureter as it descends into the pelvic cavity. These changes may be influenced by several factors. The enlarged uterus physically contributes to ureteral compression. The upper ureter also develops increased tone during pregnancy because of hypertrophy of its smooth muscle and hyperplasia of surrounding connective tissue (3). These factors may also contribute to urinary tract infection (UTI) by increasing urinary volume within the collecting system and by increased stasis (see Urinary Tract Infections, p. 816).
Understandably, there are very limited renal biopsy data from any normal populations without renal disease, and in particular of normal pregnant women. No microscopic morphologic changes are usually observed in the kidney during normal pregnancy (1). Endotheliosis, a term used to describe the characteristic widespread endothelial cell swelling seen in preeclampsia/eclampsia (see Preeclampsia and Eclampsia, p. 819), may be present in very segmental areas even in normal pregnancy. In contrast, a small study in 1960 investigated five healthy pregnant controls, none of whom showed signs of endotheliosis (4).
Thus, the presence of endotheliosis may not be pathognomonic for preeclampsia; rather, its extent may be what distinguishes the pathologic condition (5).
Thus, the presence of endotheliosis may not be pathognomonic for preeclampsia; rather, its extent may be what distinguishes the pathologic condition (5).
Functional Changes in Pregnancy
Pregnancy is a volume-expanded condition, with increased circulating volume and interstitial volume and apparent resetting of volume-sensitive receptors to sense this expansion as normal. Thus, in normal pregnancy, blood pressure decreases despite increased volume as a result of decreased peripheral resistance, even though cardiac output increases by 30% to 50% by the end of the second trimester (1). Effective renal plasma flow increases 60% to 80% during pregnancy, with slightly less increase, by about 50%, in the glomerular filtration rate (GFR) (1). Direct micropuncture measurements in animals during pregnancy show moderate renal vasodilatation with similar reduction in resistances of afferent and efferent arterioles; thus, glomerular pressure is not increased. Direct measurements of glomerular pressures are not possible in humans. However, functional studies based on dextran sieving in normal and preeclamptic pregnancy have shown that increased filtration in late pregnancy was associated with increases in renal plasma flow and in the ultrafiltration coefficient, Kf, whereas in preeclamptic late pregnancy, there was a loss of permselectivity with accompanying decreases in Kf and renal plasma flow (6). Functional assessment with computation of Kf was also done in another study of patients immediately postpartum and the 2nd week after birth. Even at postpartum day 1, there was marked glomerular hyperfiltration, with data suggesting that decreased glomerular capillary oncotic pressure was the main determinant of this change. These changes resolved largely by postpartum week 2 with increases in oncotic pressure to supranormal levels, and thus, GFR was then only modestly elevated. Theoretical analysis suggested that transcapillary hydraulic pressure and/or increased Kf would have to be present to account for this persistent hyperfiltration (7). In an additional group of pregnant women studied in late pregnancy and 4 months postpartum, increased GFR was contributed to both by increased renal plasma flow and decreased glomerular oncotic pressure, with calculated increased Kf (8).
Possible hormonal mediators of the gestational increase in the GFR include primary gestational hormones (progesterone). However, progesterone, administered exogenously in pregnant animals, has no direct influence on renal hemodynamics. Other candidate vasoactive hormones that may change and affect renal vasodilatation include prostaglandins, the renin-angiotensin system, atrial natriuretic peptide, relaxin, and endothelin. Conclusive evidence is lacking to implicate specific mediators of altered renal hemodynamics in pregnancy.
Tubular function is also altered during pregnancy. Sodium retention occurs gradually over the course of pregnancy. Although the precise site of action has not been defined, this appears to be due largely to increased distal nephron reabsorption (1). The exact mechanisms underlying the increased retention of sodium have not been delineated, although effects of increased estrogen, placental lactogen, prolactin, growth hormone, desoxycorticosterone, renin-angiotensin, and aldosterone may contribute (1). Effects on potassium are dissociated from these effects on sodium, and tubular potassium loss is not excessive.
Proteinuria is increased in normal pregnancy, with increase in nondiscriminatory shunt pathways, that is, pathways that allow passage of charged, noncharged, and variably sized molecules, and an upward shift in pore size distribution deduced by dextran-sieving data (8). Proteinuria greater than 0.3 g/24 hours or urine protein:creatinine ratio ≥0.3 is regarded as abnormal during pregnancy by American Society of Hypertension guidelines (9).
Hematuria occurs frequently in normal pregnancy. In a case-control prospective study of 902 women, 20% had dipstick-positive hematuria on at least two occasions during pregnancy. There was no increased development of preeclampsia, gestational hypertension, or small-for-gestational-age baby in those with versus those without hematuria. Thus, transient hematuria during pregnancy seldom signifies a disorder with likely adverse effects on pregnancy (10).
Glycosuria also occurs commonly in pregnancy and returns to normal within 1 week postpartum (1). This finding appears related to change in tubular function and does not necessarily reflect glucose intolerance. The increased glucose in urine may facilitate development of bacteriuria (see section Incidence and Risk Factors for Bacteriuria). Tubular reabsorption of uric acid is decreased early in pregnancy and leads to relative hypouricemia at this stage. In contrast, preeclamptic women show hyperuricemia (see Preeclampsia and Eclampsia, p. 819). Thus, an increased serum uric acid level is a useful indicator of preeclampsia early in pregnancy. However, uric acid reabsorption increases normally in the third trimester, and serum levels then approach or exceed nonpregnant levels (1). Hypercalciuria also occurs commonly in pregnancy, yet urolithiasis is uncommon. This phenomenon may be due to the accompanying increase of nephrocalcin, a protein that can inhibit urinary crystallization. Additional factors that contribute to a decreased risk for calcium stone formation in pregnancy include increased citrate and magnesium excretion (1).
URINARY TRACT INFECTIONS
Incidence and Risk Factors for Bacteriuria
Significant numbers of bacteria can be found in urine cultures in patients without clear clinical manifestations of UTI. This situation occurs particularly in women and has been called asymptomatic, or covert, bacteriuria. Its importance lies in its relation to overt UTI and in particular to the associated risks in pregnancy for the mother and fetus. In pregnancy, the prevalence of asymptomatic bacteriuria ranges from 2% to 10% (11). This prevalence is comparable to the rate in sexually active nonpregnant women of reproductive age. Thus, pregnancy does not necessarily predispose to the development of asymptomatic bacteriuria, at least in developed countries (see later this section). Rather, it is possible that detection of this asymptomatic condition is increased during pregnancy.
The incidence of bacteriuria during pregnancy hits a peak between the 9th and 17th weeks of gestation. The dilation of the collecting system that begins as early as the 7th week (see p. 815) is thought to contribute to the development of upper UTI, because infection localizes to the dilated side in bacteriuric women with unilateral ureteral and calyceal dilation (2,11). Both increased urine volume within the dilated collecting system and increased urinary stasis (see p. 815) likely contribute to this increased risk of infection.
Increased frequency of asymptomatic bacteriuria is seen with a history of previous UTI, increased sexual activity, diabetes, and structural abnormalities of the urinary tract. Some,
but not all, studies suggest that age, parity, race, and sickle cell trait are also linked to increased asymptomatic bacteriuria (11). In contrast to a 6% prevalence of asymptomatic bacteriuria in pregnancy in otherwise healthy women, the rate was 12.2% in diabetic women and 18.7% in women with a history of previous UTI (11). Recent observational studies show constant and similar rates of asymptomatic bacteriuria in pregnancy in developing countries and in developed countries. The prevalence of asymptomatic bacteriuria in pregnancy is increased in patients with low socioeconomic status, but the mechanisms underlying these observations are unknown (11).
but not all, studies suggest that age, parity, race, and sickle cell trait are also linked to increased asymptomatic bacteriuria (11). In contrast to a 6% prevalence of asymptomatic bacteriuria in pregnancy in otherwise healthy women, the rate was 12.2% in diabetic women and 18.7% in women with a history of previous UTI (11). Recent observational studies show constant and similar rates of asymptomatic bacteriuria in pregnancy in developing countries and in developed countries. The prevalence of asymptomatic bacteriuria in pregnancy is increased in patients with low socioeconomic status, but the mechanisms underlying these observations are unknown (11).
Gestational glycosuria and decreased potassium stores may facilitate bacterial growth in urine (2). Although not peculiar to pregnancy, the virulence factors of organisms and the status of the host’s uroepithelium are clearly important. The formation of a biofilm and bacterial ability to adhere to and invade the urothelium are key for UTI occurrence. Virulent uropathogenic Escherichia coli (UPEC) typically adhere and activate host responses, including an innate immune response and Toll-like receptor signaling. Type 1 fimbriae bind to various epitopes, including Tamm-Horsfall glycoprotein mannosylated sites, secretory IgA, or uroplakins in the bladder urothelium. P fimbriae mediate mannose-resistant adherence of UPEC and are associated with acute pyelonephritis (12). When bacteria adhere via P fimbriae, the innate immune response is activated with increased Toll-like receptor 4 signaling and cytokine and neutrophil recruitment. Patients with decreased or defective Toll-like receptor 4 may thus have asymptomatic bacteriuria. Genetic variations in these response elements may thus favor asymptomatic bacteriuria versus acute pyelonephritis as outcomes of bacteria in the urine (13).
The most common organism causing bacteriuria during pregnancy is E. coli, followed by other gram-negative bacteria (Klebsiella sp, Enterobacter sp., Proteus sp.). Other reported organisms include Staphylococcus saprophyticus, which promotes stone formation as well. If fastidious organisms such as Ureaplasma urealyticum and Gardnerella vaginalis are also selected for by culture conditions, up to 25% of pregnant patients may show bacteriuria. However, these latter organisms have not been shown to play a pathogenic role (14).
Asymptomatic or Covert Bacteriuria
Effects on the Kidney
Other kidney abnormalities may develop or may be detected in patients with asymptomatic or covert bacteriuria during pregnancy (15,16,17,18,19,20,21). First is the issue of underlying structural urologic abnormalities or evidence of chronic pyelonephritis. Pregnant patients with asymptomatic bacteriuria showed a high prevalence of urinary tract abnormalities in a series of patients with urologic studies performed postpartum, also observed in a more recent study where only 14.3% of pregnant women with acute pyelonephritis showed normal kidneys by ultrasound (15). Ten percent of these patients showed evidence of chronic pyelonephritis (14). In several large series of pregnant women with asymptomatic bacteriuria who were followed for 2 to 14 years (14), bacteriuria was present in 16% to 29% during follow-up, and radiologic evidence of chronic pyelonephritis was seen in 9% to 29%. Many patients had underlying abnormalities, including bifid pelvis and ureteric duplication, and 27% showed signs of chronic pyelonephritis, such as calyceal blunting, diminished cortical thickness, and irregular renal contour. Despite these significant abnormalities, long-term follow-up of patients with asymptomatic bacteriuria during pregnancy has shown only rare cases of deterioration of renal function (20,21).
Assessment of these various series is difficult for several reasons. First is the danger of considering bacteriuria of pregnancy a specific complication acquired during pregnancy. The appreciable prevalence of asymptomatic bacteriuria in children and nulliparous women raises the possibility that asymptomatic bacteriuria may be present before conception. Bacteriuria was present in 8% of nulliparous married women compared with 6.6% of pregnant women (19). Many girls with asymptomatic bacteriuria have bacteriuria when they grow up and become pregnant. From 40% to 64% of women with a history of asymptomatic bacteriuria during childhood have bacteriuria during pregnancy (20,21). Further, it is difficult to assess whether asymptomatic bacteriuria is detrimental to kidney function because of the lack of prepregnancy measurements of renal function or serial radiographic studies. In addition, the radiologic abnormalities described in the previous paragraph in many patients with asymptomatic bacteriuria could indicate a predisposition to infection or a consequence of infection, or they may even be unrelated to infection. Whether the renal scars detected radiologically reflect injury from bacteriuria occurring in adulthood or from childhood UTI also is not clear.
Second is the potential of asymptomatic bacteriuria to cause acute pyelonephritis and its associated complications, including preterm labor, low birth weight, and growth retardation. The incidence of acute pyelonephritis in a recent large study of pregnant women was 0.07%, less than that observed before routine screening for asymptomatic bacteriuria and treatment were instituted (11). Bacteriuria in pregnancy leads to acute symptomatic infection in 20% to 40% of patients (14,15). Conversely, women without bacteriuria early in pregnancy rarely develop symptomatic urinary tract disease. Successful treatment of bacteriuria largely prevents the development of acute pyelonephritis. The consequences of symptomatic UTI are discussed below.
Effects on Pregnancy
The effect of asymptomatic bacteriuria on pregnancy outcome was previously controversial. Higher rates of prematurity and intrauterine growth retardation in patients with asymptomatic bacteriuria were observed in older studies but were initially not uniformly confirmed (15). Recent large studies demonstrate an effect of asymptomatic bacteriuria on pregnancy with increased preterm delivery, intrauterine growth retardation, and low birth weight (15).
Symptomatic Urinary Tract Infection and Pyelonephritis
Consequences and Complications
The exact extent of the urinary tract that is involved by infection cannot be readily assessed without invasive tests. Therefore, the term symptomatic bacteriuria is used by many authors to encompass both acute pyelonephritis and symptomatic infection of the lower urinary tract. Although risk factors for bacteriuria may overlap with those in asymptomatic infection, as discussed in Asymptomatic or Covert Bacteriuria, the consequences differ. Symptomatic UTI has well-defined serious consequences during pregnancy. Acute pyelonephritis in pregnancy is associated with bacteremia in 10% and endotoxic shock in up to 3% of patients (15).
The ability to predict which patients with asymptomatic bacteriuria will develop symptomatic infection is not established. This inability relates in part to a lack of understanding of the mechanisms that allow asymptomatic bacteriuria to become symptomatic, presumably reflecting upper UTI in most patients. A history of previous symptomatic UTI greatly increases the risk of progressing from asymptomatic to symptomatic infection during pregnancy (15). Underlying abnormalities, such as reflux, may predispose to progression from asymptomatic to symptomatic infections. Despite the dilation of the collecting system that normally occurs in pregnancy, reflux is uncommon, detected in only 0% to 3%, and likely does not play a significant role in the development of symptomatic infection in most patients. However, the vast majority of patients, over 85%, with pyelonephritis during pregnancy had abnormal renal ultrasound, including, for example, hydroureter, hydronephrosis, or renal calculi, with normal ultrasound in only 14.3% (15). Acute pyelonephritis complicated 6% of pregnancies in patients with reflux in one series (16). Successful ureteral reimplantation in women with reflux and past symptomatic UTI did not, however, abolish complications in subsequent pregnancies: 57% had UTI, and 17% had pyelonephritis during later pregnancies (17). These findings indicate that reflux cannot be regarded as the only mechanism for development of upper UTI in these patients. It is possible that the apparent greater ease of ascending infection in pregnancy is due to mechanical changes in pregnancy and altered innate immune responses.
The importance of preceding asymptomatic infection in the development of symptomatic infection has been clearly demonstrated. Screening programs to detect and treat asymptomatic bacteriuria in pregnancy decrease the incidence of pyelonephritis (15). This effect was shown dramatically by Kincaid-Smith and Bullen (22), who noted a 3.3% prevalence of symptomatic bacteriuria in a treated group of patients with asymptomatic bacteriuria versus a prevalence of 36.6% in a placebo-treated group.
The prevention of pyelonephritis during pregnancy has important implications for subsequent pregnancies as well. Patients treated for acute pyelonephritis have frequent occurrences of symptomatic UTI, both later in pregnancies and when not pregnant. This situation may result from increased susceptibility to repeated infection once parenchymal scarring has occurred. Radiologic findings of chronic pyelonephritis were present at follow-up in 14% to 27% of women who had bacteriuria detected during pregnancy (16,18). Women with renal scars resulting from childhood UTI had a higher incidence of bacteriuria during pregnancy than when scarring was absent (47% vs. 27%) (19). Patients with renal scars also had a significantly increased relative risk of hypertension, preeclampsia (up to 7.6-fold increase), and fetal morbidity during subsequent pregnancies compared with controls (20,21). In contrast, patients with vesicoureteral reflux but without scars had no increased risk of gestational hypertension or preeclampsia, although the risk of UTI was increased. This risk was not changed by ureter reim-plantation (20). However, whether pregnancy affects the course of the underlying condition or merely increases the detection of infection and renal scarring has not been proven.
Symptomatic UTI has serious consequences for the pregnancy as well as for the mother. Prematurity occurred in 20% of pregnant women with acute pyelonephritis. There is controversy whether treated pyelonephritis also has adverse pregnancy effects (15). Older data showed that the fetal mortality rate was increased, but this was not confirmed in more recent studies (11). Production of phospholipase A2 by the infecting organism may contribute to preterm labor by liberating arachidonic acid esters from phospholipids of infected amnionic and chorionic membranes, thus increasing levels of prostaglandins E2 and F2, which can trigger labor (11). A role for cytokines induced by infection, such as tumor necrosis factor and cachectin, has been suggested in preterm labor (23).
An additional serious complication of pyelonephritis in pregnancy is the occurrence of pulmonary insufficiency resembling adult respiratory distress syndrome, estimated to occur in about 7% of pregnant patients with acute pyelonephritis (11). This pulmonary injury may be related to endotoxin, prompted by lysis of bacteria in response to treatment.
Treatment
Cost-effectiveness and cost-to-benefit analyses show that screening for and treatment of asymptomatic bacteriuria prevents pyelonephritis in pregnancy and decreases preterm delivery (11). Currently, screening cultures are recommended for all pregnant women, preferably during the 16th gestational week for greatest potential impact on pregnancy outcome. Once asymptomatic bacteriuria is detected and treated, urine cultures should be repeated monthly throughout pregnancy, because as many as one third will have relapse or recurrence during pregnancy. If more than one relapse occurs, intravenous pyelography is recommended after 6 weeks postpartum. Urologic evaluation during pregnancy has been recommended if asymptomatic bacteriuria recurs or if appropriate treatment fails to eradicate bacteriuria. Either setting indicates a possible underlying urologic anomaly, obstruction, or abscess. Treatment of pyelonephritis is approached in a similar manner to that in nonpregnant patients.
HYPERTENSIVE DISORDERS OF PREGNANCY
Hypertension is a common problem during pregnancy, affecting 6% to 8% (24). Hypertension in pregnancy is defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg (24). It may be preexisting, or caused by, or exacerbated by the pregnancy. These complexities have led to difficulties in establishing the causes of hypertensive conditions in pregnancy. Although many classifications have been proposed, this discussion is based on the classification initially proposed by Lindheimer and Katz and slightly modified by the National High Blood Pressure Education Program Working Groups on High Blood Pressure in Pregnancy (as reviewed in reference (25). These disorders are divided into chronic hypertension, gestational hypertension, preeclampsia superimposed on chronic hypertension, and preeclampsia or eclampsia.
Chronic Hypertension
The most common cause of hypertension during pregnancy is preeclampsia (see p. 819), followed by essential hypertension and secondary causes, among which renal disorders are the most common. The prevalence of chronic hypertension in pregnancy is about 3% in the United States (25,26). In patients who develop hypertension, but not preeclampsia during pregnancy, underlying renal disease should be considered (see below).
Not surprisingly, populations with a higher general incidence of hypertension also show a higher incidence of hypertension detected during pregnancy. Thus, in a study from South Africa, black women showed a higher prevalence of hypertension during pregnancy than that seen in other predominantly white populations: 23% versus 7% to 10% (27).
Not surprisingly, populations with a higher general incidence of hypertension also show a higher incidence of hypertension detected during pregnancy. Thus, in a study from South Africa, black women showed a higher prevalence of hypertension during pregnancy than that seen in other predominantly white populations: 23% versus 7% to 10% (27).
Although pregnant women with mild essential hypertension do have a greater risk of developing superimposed preeclampsia, most of these patients do not experience complications during pregnancy. Treatment of hypertension has not been shown to decrease risk for preeclampsia (25). If hypertension in pregnancy is associated with proteinuria greater than 500 mg/d, even in the absence of overt preeclampsia, increased maternal complications and worse fetal outcome are seen (28).
Poor outcomes and even death may occur when hypertension is secondary to scleroderma, cocaine ingestion, or pheochromocytoma. Fibromuscular dysplasia is a frequent cause of renal artery stenosis in young women, and it should be considered when hypertension precedes pregnancy. An apparent paradox is observed in patients with renal artery stenosis: they may actually have reduced hypertension during pregnancy because altered tubular function in pregnancy leads to less potassium loss and less induction of aldosterone (29). Correction of the stenosis by angioplasty with resolution of hypertension decreases risk of fetal and maternal complications (30).
In patients with chronic hypertension without superimposed preeclampsia, most (more than 85%) experience uncomplicated pregnancies. However, birth weights are lower and the perinatal mortality rate is increased in patients with hypertension due to underlying renal disease or other secondary cause, older than 40 years, diabetic, with previous pregnancy loss, or history of stroke compared with normotensive patients (25). Whether treatment of mild chronic hypertension affects the risks of premature delivery and superimposed preeclampsia is controversial. Methyldopa has been recommended as the drug of choice. The angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, which are widely used in many other settings of hypertension and cardiovascular disease, are contraindicated in pregnancy because of their association with both neonatal acute renal failure and birth defects (26).
Preeclampsia Superimposed on Chronic Hypertension
Patients with this disorder have underlying hypertension in combination with a further elevation of blood pressure and the appearance of or increase in proteinuria, that is, preeclampsia. Edema may also develop. In a series of 13 women with preexisting essential hypertension or renal disease who were suspected of having superimposed preeclampsia clinically, only 7 of the biopsies showed typical changes of preeclampsia (31). About 25% of pregnant women with chronic hypertension develop superimposed preeclampsia (25). Preeclampsia in this population frequently occurs in midpregnancy or early in the third trimester. An acute medical emergency can arise in this setting, and fetal outcome may be jeopardized (25).
Gestational Hypertension
Gestational hypertension is defined as hypertension after 20 weeks estimated gestation in women without previous hypertension and without proteinuria (25). In various studies, 15% to 45% of these patients developed preeclampsia (25). Blood pressure normalizes within 10 days after delivery, but late hypertension may recur in subsequent pregnancies (29).
Preeclampsia and Eclampsia
Clinical Findings
Toxemia of pregnancy, including both preeclampsia and eclampsia, occurs in up to 8% of pregnancies, with an increasing incidence, and especially higher incidence in developing countries (32,33). True eclampsia, defined as the occurrence of convulsions in association with the signs and symptoms of preeclampsia, was found in nearly 4.9 per 10,000 pregnancies in one study from the United Kingdom (34). In lower-income settings, 2.3% of women with preeclampsia developed eclampsia, compared to only 0.8% of women in countries with higher income (35). The disease is named eclampsia (from the Greek eklampsis, meaning sudden flashing, i.e., lightening) because of the sudden occurrence of onset of convulsions in association with the signs and symptoms of preeclampsia. Preeclampsia is primarily a disease of the nullipara and manifests usually after the 20th week of the first gestation. Before the 20th week of gestation, preeclampsia is most frequently associated with molar pregnancy or its degeneration. When the disease occurs for the first time in multiparas, it is typically associated with multiple-birth gestation, fetal hydrops, preexisting vascular disease, or renal disease (36). A large study systematically reviewing controlled studies published from 1966 to 2002 examined unadjusted relative risk for development of preeclampsia based on factors that could be determined at the initial visit. Increased risk of preeclampsia was seen in patients with a history of previous eclampsia, antiphospholipid antibodies, preexisting diabetes, multiple pregnancy, nulliparity, family history of preeclampsia, increased diastolic blood pressure at initial visit, increased body mass index before pregnancy or even at initial visit, and maternal age of 40 years or older. There was also an increased risk with an interval of 10 years or more since a previous pregnancy, with autoimmune disease, renal disease, and chronic hypertension (37).
Preeclampsia is characterized by hypertension (greater than 140/90 mm Hg or marked increase over baseline), proteinuria, and edema, especially of face and hands. The labile hypertension and altered circadian rhythm of blood pressure in preeclampsia, with higher levels at night, may lead to difficulty in detecting the increased blood pressure if blood pressure is measured only during the day (1). Wide fluctuations in blood pressure, even including the normal range, are thought to reflect increased sensitivity to vasoconstrictors (7). Proteinuria is usually not marked, but the nephrotic syndrome with protein loss of up to 23 g/d has been reported (38). The condition occasionally progresses to a convulsive phase, termed eclampsia, which may be life threatening. Eclampsia occurs in about 5 of 10,000 live births. The incidence has declined, perhaps due to urgent delivery in the preeclamptic patient or use of magnesium sulfate (39). Preeclampsia and eclampsia follow only hemorrhage as a cause of pregnancy-associated death in women worldwide (35). Data from the United Kingdom showed that nearly 2% of eclamptic women died, as did 7% of their offspring (34). Eclampsia may also develop without an obvious preceding stage of preeclampsia (34). In some patients, hypertension, proteinuria, and convulsions may occur in the immediate postpartum period (so-called “late postpartum eclampsia”). Preeclampsia and eclampsia may occur de novo,
or they may be superimposed on preexisting hypertensive disorders, as mentioned below.
or they may be superimposed on preexisting hypertensive disorders, as mentioned below.
In preeclampsia, the GFR is decreased, but this change may not be detectable because of the preceding normal increase of GFR seen with pregnancy. Detailed studies have now elucidated further the functional implications of the endotheliosis lesion that characteristically occurs in preeclampsia/eclampsia. Patients with preeclampsia had markedly decreased GFR levels, 91 ± 23 versus 149 ± 34 mL/min/1.73 m2 in normals, whereas renal plasma flow and oncotic pressures were similar to that in normal pregnancy. Combined physiologic and morphometric studies were used to estimate the glomerular ultrafiltration coefficient (Kf) in normal pregnancy and preeclampsia. The decrease of both density and size of endothelial fenestrae and the substantial subendothelial accumulation of lucent material were postulated to lower glomerular hydraulic permeability in preeclampsia. The presence of cellular interposition, that is, infiltrating cells, usually monocyte/macrophages, interposed between the endothelium and the GBM, also was postulated to contribute to decrease in effective filtration surface area, so that single nephron Kf estimated in this matter was well below control. These changes occurred despite significantly larger glomerular volume in preeclampsia so that actual filtration surface area was reduced to only a minor extent (40). These morphologic findings suggest that structural lesions could be a major contribution to decreased GFR owing to the decrease in Kf and that hemodynamic changes may have less influence than previously thought (33).
Hyperuricemia resulting from decreased clearance predates heavy proteinuria in nearly all cases of preeclampsia. Sodium is retained, contributing to edema. Peripheral resistance and vascular sensitivity to angiotensin II are increased (41). This finding is in contrast to the blunted responsiveness to angiotensin in normal pregnancy with up-regulation of all renin-angiotensin components. The Ang (1,2,3,4,5,6,7) metabolite of angiotensin II counteracts many of angiotensin II’s actions and is increased in normal pregnancy, in contrast to a decrease in preeclampsia (41). Red blood cell fragmentation may occur even in the absence of the postpartum hemolytic-uremic syndrome (see Chapter 18). Platelet counts are decreased, whereas fibrin degradation products and fibronectin levels in plasma are increased, indicative of fibrinolysis and vascular injury (42). Despite the vascular injury, serum complement levels are usually not different in normal and preeclamptic pregnancies (43). Urinalysis is nonspecific and may show occasional red blood cells, white blood cells, and casts.
Differential Diagnosis
Because the clinical findings of preeclampsia are largely nonspecific, misclassification occurs commonly. Thus, several studies of postpartum renal biopsies in women thought to have preeclampsia clinically showed other renal lesions in as many as half these patients (38,40,44,45). Underlying renal biopsy lesions included chronic glomerulonephritis, tubulointerstitial lesions, membranous glomerulopathy, sickle cell nephropathy, acute poststreptococcal glomerulonephritis, minimal change nephrotic syndrome, and diabetic nephropathy, in descending order of frequency. In some patients, lesions of preeclampsia were superimposed on specific findings of these renal diseases (31,46). The clinical diagnosis is inaccurate even in a large proportion of primiparas: 25% in one series of patients with clinically diagnosed preeclampsia actually had chronic glomerulonephritis (47). In patients with apparent preeclampsia before the third trimester, an especially high prevalence of underlying disease has been found. When preeclampsia was diagnosed clinically before 37 weeks’ gestation, 67% of patients in one study had disease other than preeclampsia, including glomerulonephritis (IgA nephropathy, other mesangial glomerulonephritis, reflux nephropathy, polycystic kidneys, and diabetes) and essential hypertension (48).
The effect of pregnancy on preexisting renal disease is discussed below on page 837. These patients represented a population referred for evaluation of possible renal disease postpartum, and many had hematuria. In one study, women with clinical diagnoses of either preeclampsia or gestational hypertension without clinical evidence of underlying disease during pregnancy were evaluated postpartum for evidence of renal disease. This clinical evaluation showed evidence of underlying renal disease in only 7 of the 87 (8%) women with a diagnosis of preeclampsia and in 16 of the 99 (16%) patients with apparent gestational hypertension (49). However, renal biopsy was performed only in the single patient who had hematuria in this series (demonstrating thin basement membrane lesion). Two points emerge from these studies. First, the incidence of underlying renal disease varies among patients with apparent preeclampsia, depending on patient referral and selection criteria. Second, the exact structural lesions underlying renal dysfunction in pregnancy cannot be known precisely in the absence of renal biopsy.
Nonrenal disorders may also be associated with preeclampsia-like syndromes. A case report describes the occurrence of a reversible preeclampsia-like syndrome during pregnancy in a hypothyroid patient (50). Renal biopsy showed enlarged, bloodless glomerular tufts with endothelial cell swelling and mesangial interposition, similar to findings seen in hypothyroidism without pregnancy and to the typical lesions of preeclampsia (see Pathologic Changes, pp. 821-825).
Course and Prognosis
Clinical signs and symptoms of preeclampsia and eclampsia resolve with cessation of pregnancy, typically within 24 hours of delivery. If the patient is near term, preeclampsia is best treated by induction of labor. If the fetus is immature, treatment with bed rest and sedation may allow continuation of the gestation unless findings of HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome (see HELLP syndrome, p. 832) are present. Intravenous magnesium sulfate is used for impending eclampsia because of its antihypertensive effects and amelioration of central nervous system symptoms of preeclampsia and eclampsia (1). Other treatment strategies focus on antihypertensives and fluid and electrolyte management. However, the basic nature of volume homeostasis in preeclampsia has not been defined. Some investigators view this as a condition with volume overload, whereas others point to evidence of decreased plasma volume in preeclampsia. Thus, diuretics and volume expansion have variously been advocated in the treatment of preeclampsia.
Hypocalcemia is observed in preeclampsia, and treatment of preeclamptic patients with calcium contributes to normalization of blood pressure. Prophylactic calcium supplementation also reduced the risk of gestational hypertension and preeclampsia, especially in developing countries, or in developing countries in women with low basal calcium
levels (51). Increased emphasis on the possible participation of thromboxane and prostacyclin in the pathogenesis of vasospasm and thrombocytopenia has led to several trials of low-dose aspirin for the prevention of preeclampsia. However, a specific, effective prophylactic approach has not been defined.
levels (51). Increased emphasis on the possible participation of thromboxane and prostacyclin in the pathogenesis of vasospasm and thrombocytopenia has led to several trials of low-dose aspirin for the prevention of preeclampsia. However, a specific, effective prophylactic approach has not been defined.
The consequences of preeclampsia and eclampsia are difficult to assess because of several factors. First, clinical diagnosis of preeclampsia is not accurate, as discussed in Differential Diagnosis (p. 820). Therefore, the long-term consequences of preeclampsia may reflect the course of underlying disease and not the pregnancy-induced injury. Second, clinical parameters are not sensitive indicators of renal injury, and long-term follow-up is necessary to realize fully the impact of an injury on progressive renal dysfunction. Finally, limited renal biopsy studies have been performed. Although definitive diagnosis of preeclampsia can be achieved only with renal biopsy, most clinicians do not advocate this procedure in this setting. To complicate matters further, the timing of biopsies in relation to the preeclampsia-associated injury varies greatly. Furthermore, even normal pregnancies may be associated with mild focal endotheliosis lesions in biopsies (5). Even heavy proteinuria typically resolves by 3 months postpartum, and the typical glomerular lesion of preeclampsia, that is, endothelial swelling, also appears to be completely reversible (see Pathologic Changes, below). In contrast, persistent proteinuria, hypertension, and abnormal urinalysis findings beyond 3 months postpartum suggest other underlying renal disease (see Differential Diagnosis) (24). Long-term, preeclampsia is linked to increased cardiovascular disease and increased chronic kidney disease (39). When preeclampsia only occurred in the first pregnancy, the relative risk for end-stage renal disease was 4.7, with further increase if preeclampsia developed in additional pregnancies (52). Severe preeclampsia is also associated with doubling of the risk of development of venous thrombosis compared to normotensive pregnancies. The risk of development of type 2 diabetes mellitus is also increased in women with a history of preeclampsia, even after controlling for other risk factors for development of diabetes (39).
Renal biopsy findings correlated with some of these late complications. When nephrosclerosis, that is, sclerosis of vessels and glomeruli, was present in the renal biopsy in preeclamptic patients, 74% of patients had hypertension on follow-up (53). In contrast, only 9.4% of preeclamptic patients without vascular lesions developed hypertension, not significantly different from age-, sex-, and race-matched control populations. These data were taken to suggest that persistent hypertension after preeclampsia reflected preexisting disease and was not a consequence of preeclampsia. However, because baseline biopsies are typically not available, this issue has not been directly proven. Limited studies with follow-up biopsies have directly examined the reversibility of renal lesions in preeclamptic patients. In a small study of patients with nephrosclerosis detected in immediate postpartum biopsies, follow-up biopsies revealed persistent arteriolar changes, and four of these patients developed hypertension again in subsequent pregnancies (54). Taken together, these data suggest that arteriolar sclerosis may be irreversible and associated with increased risk for subsequent hypertension. Furthermore, based on the nature of the morphologic changes, the presence of arteriosclerosis or arteriolosclerosis in patients with preeclampsia likely reflects incipient hypertension-attributed nephrosclerosis.
Pathologic Changes
HISTORICAL PERSPECTIVE
The glomerular changes of preeclampsia in autopsy specimens were initially described in detail by Löhlein in 1918 (55) and a few years later by Fahr (56,57). These investigators noted glomerular tuft swelling and expansion of the glomerular capillary wall, resulting in a bloodless appearance of the capillaries and capillary lumen occlusion. Subsequently, Bell (58) suggested that basement membrane thickening was responsible for the capillary occlusion. Sheehan (59) reported an extensive autopsy experience of patients with toxemia who died of apparent incidental obstetric complications. Remarkably, most of these autopsies were performed within 15 minutes to 2 hours after death, thereby avoiding artifacts from extensive autolysis (59,60). Sheehan, with only light microscopic studies available, noted glomerular endothelial cell swelling and fibrils between the endothelial cells and the basement membranes and postulated that endothelial cell changes accounted for the thickened capillary wall. Dieckmann et al. (61) were among the first to examine renal biopsy material in preeclampsia, and they confirmed many autopsy findings. These early light microscopic studies focused on the thickened glomerular capillary wall, thought to represent a thickened glomerular basement membrane. However, not until electron microscopic examination became available were these light microscopic observations further elucidated. Spargo et al. (62) and Farquhar (63) demonstrated by electron microscopy that the thickening of the glomerular capillary wall seen by light microscopy was not due to the glomerular basement membrane thickening in that the lamina densa of the glomerular basement membrane consistently was normal. However, these ultrastructural studies confirmed the presence of endothelial cell swelling. The term glomerular endotheliosis was coined by Spargo et al. for the described lesion (62). Other investigators confirmed the presence of glomerular endothelial cell swelling (64,65,66,67) and also described swelling of the podocytes (66,67). The occurrence of substantial glomerular subendothelial deposits visualized by electron microscopy was noted by Hopper et al. in 1961 (68). These deposits and the presence of a translucent subendothelial zone, possibly relating to fibrin deposition, were described in detail by Kincaid-Smith in 1973 (69). Furthermore, cellular interposition, that is, interposed portions of cells, most often monocyte/macrophages, between the endothelium and the GBM, was recognized to contribute to glomerular capillary wall thickening by Altchek (64) and Ishikawa (70).
GROSS APPEARANCE
Judging by the older reports on autopsies in cases of eclampsia, the kidneys show no distinctive changes visible to the naked eye (71). They are of normal size or are slightly enlarged; the cortex is pale and widened in the larger kidneys, whereas the glomeruli can often be seen to be unduly prominent and gray if looked at with a hand lens.
MICROSCOPIC FINDINGS
The renal lesions are not substantially different in preeclampsia and eclampsia. The severity of morphologic alterations, which primarily affect the glomeruli, parallels the severity of clinical disease.
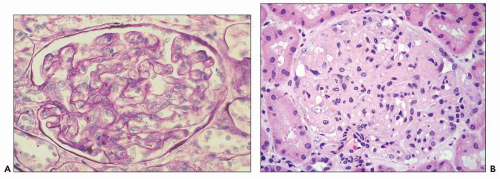
Glomeruli The glomeruli are diffusely slightly enlarged and swollen, and they appear bloodless (Fig. 19.1). In one postmortem study (71), glomerular size was approximately 10% larger
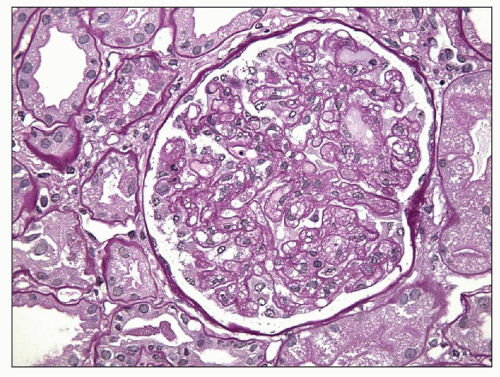
than normal. The glomeruli show a characteristic lobular pattern from capillary expansion producing cigar-shaped lobules. The glomerular capillary lumina are narrowed or even obstructed because of marked mesangial and endothelial cell swelling and hypertrophy, so-called glomerular capillary endotheliosis (62). The glomerular capillary loops are dilated, so-called ballooning, particularly at the tubular pole. This may result in herniation of the glomerular tuft into the proximal tubule (so-called pouting; Fig. 19.2) (71). This extension involved about half of the glomeruli in this study (71), with capillary loops extending on average 50 µm into the proximal tubule. Glomerular capillary endotheliosis has been viewed by Gaber et al. (72) as pathognomonic for preeclampsia. Although it is well accepted that this lesion is part of the glomerular structural changes in preeclampsia, and the fully developed lesion as assessed by light and electron microscopy is characteristic, other lesions also occur in varying proportion, depending on the timing of the biopsy and the severity of the disease. Finally, endotheliosis-like lesions may not be uniquely present in preeclampsia. Normotensive patients with abruptio placentae showed lesions similar to, albeit milder than, those described in this section (73). Furthermore, in a study of renal biopsies performed 8 to 10 days postpartum in 32 women with gestational hypertension without proteinuria or clinical evidence of preeclampsia, 12 biopsies revealed the “specific” pattern of preeclampsia (74). A recent small study revealed that even some normal pregnancies may also be associated with small areas of endotheliosis-type lesions (5).
than normal. The glomeruli show a characteristic lobular pattern from capillary expansion producing cigar-shaped lobules. The glomerular capillary lumina are narrowed or even obstructed because of marked mesangial and endothelial cell swelling and hypertrophy, so-called glomerular capillary endotheliosis (62). The glomerular capillary loops are dilated, so-called ballooning, particularly at the tubular pole. This may result in herniation of the glomerular tuft into the proximal tubule (so-called pouting; Fig. 19.2) (71). This extension involved about half of the glomeruli in this study (71), with capillary loops extending on average 50 µm into the proximal tubule. Glomerular capillary endotheliosis has been viewed by Gaber et al. (72) as pathognomonic for preeclampsia. Although it is well accepted that this lesion is part of the glomerular structural changes in preeclampsia, and the fully developed lesion as assessed by light and electron microscopy is characteristic, other lesions also occur in varying proportion, depending on the timing of the biopsy and the severity of the disease. Finally, endotheliosis-like lesions may not be uniquely present in preeclampsia. Normotensive patients with abruptio placentae showed lesions similar to, albeit milder than, those described in this section (73). Furthermore, in a study of renal biopsies performed 8 to 10 days postpartum in 32 women with gestational hypertension without proteinuria or clinical evidence of preeclampsia, 12 biopsies revealed the “specific” pattern of preeclampsia (74). A recent small study revealed that even some normal pregnancies may also be associated with small areas of endotheliosis-type lesions (5).
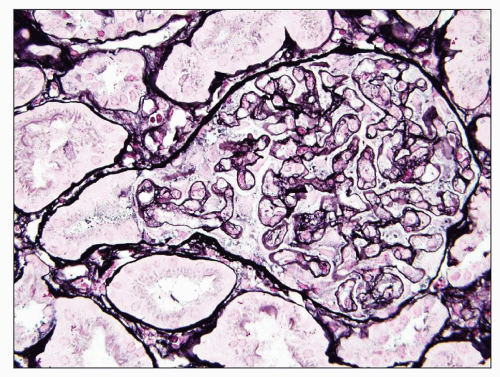 FIGURE 19.2 Glomerulus from a patient with preeclampsia showing similar features as in Figures 19.1, as well as herniation of the tip of the swollen glomerulus into the proximal tubule (so-called pouting), which is a characteristic but not specific feature of preeclampsia/eclampsia. (Jones silver stain, ×400.) (Courtesy of J. Charles Jennette, MD.) |
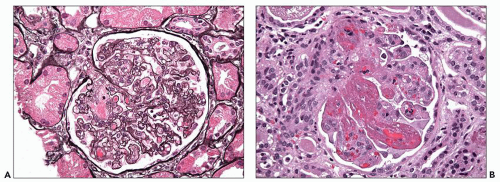
Glomerular cellularity may be normal or slightly increased with only rare, or no, neutrophils. Mesangial and endothelial cell vacuolization with accumulation of fluid and lipid is visualized well on osmium-fixed toluidine blue-stained thick sections (45). The foamy, bubbly appearance of the glomerular endothelial, or to some extent mesangial, cells, or even cholesterol clefts at later stages, may relate to severe proteinuria (Fig. 19.3) (62). Fibrils within swollen glomerular endothelial cell cytoplasm adjacent to the basement membrane may be visualized (59,60). Mesangial cells and matrix may be mildly increased, and cellular processes may extend between the glomerular basement membrane and endothelium (cellular interposition). Cellular interposition is especially prominent in more severe disease and in the healing stage (69,71,75). Podocytes are swollen and prominent and may contain hyaline droplets that are positive for periodic acid-Schiff stain (Fig. 19.4). Crescents are seen only in the most severe cases of preeclampsia and eclampsia (60,71).
The glomerular capillary wall is thickened. Its components may be distinguished by the Alcian blue/periodic acid-Schiff reaction, which stains the glomerular basement membrane magenta and the cytoplasm blue. Silver stain may show a double contour, in part because of the interposition of cell cytoplasm processes (Fig. 19.5) (69). Glomerular basement membrane remodeling is associated with increased staining for
laminin, type IV collagen, fibronectin, and proteoglycan (76). Carefully performed studies from biopsy material have revealed no distinct changes of the juxtaglomerular apparatus (77).
laminin, type IV collagen, fibronectin, and proteoglycan (76). Carefully performed studies from biopsy material have revealed no distinct changes of the juxtaglomerular apparatus (77).
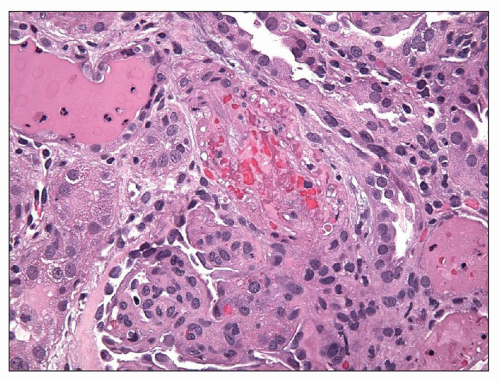
Time Course of Glomerular Lesions Endotheliosis, as described and coined by Spargo et al. (72), has often been regarded as the pathognomonic feature of preeclampsia. However, depending on the timing of the biopsy, other features may also be prominent. Various series have shown remarkable differences in frequency of detection of glomerular subendothelial deposits of fibrin or fibrinoid material (62,66,68,69,73,75). The analyses of Kincaid-Smith (75) of the evolution of lesions during pregnancy and postpartum indicate that these fibrin-like deposits may disappear quickly postpartum. Subendothelial deposits are most consistently present in biopsies done during the first few postpartum days (75). Rapid fibrinolysis can occur, and thus, fibrin would be less likely to be present after delivery and resolution of the preeclamptic injury cascade. Focal thrombotic microangiopathy may also be present in clinically severe cases of preeclampsia or full-blown eclampsia, mirroring the severity of the disease (Fig. 19.6).
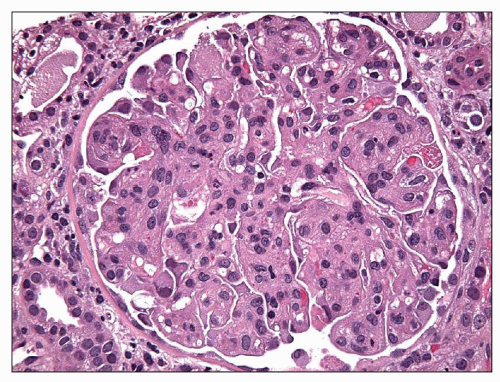 FIGURE 19.4 Glomerulus from a patient with preeclampsia revealing similar lesions as in Figure 19.3 and segmental hyaline droplets in the podocytes. (H&E, ×400.) (Courtesy of Vivette D’Agati, MD.) |
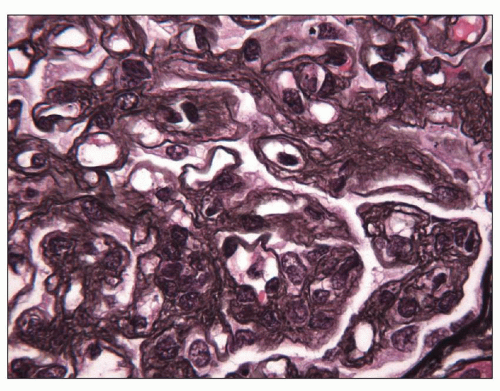 FIGURE 19.5 Glomerulus from a patient with preeclampsia showing widespread glomerular basement membrane double contours, revealed by electron microscopy (see Fig. 19.14) to be due to interposition and increased lamina rara interna. (Jones silver stain, ×1000.) (Courtesy of Vivette D’Agati, MD.) |
Similarly, the extent of foam cells is correlated with the timing of biopsy. In postpartum biopsies, glomerular endothelial and, to a lesser extent, mesangial foam cells are nearly universally present as part of the endotheliosis lesion, a finding confirmed when biopsies were carried out within 12 days postpartum (62,78). In contrast, the biopsy study conducted over a longer time course by Kincaid-Smith and Fairley (79) and the extensive autopsy experience of Sheehan (59) documented only sparse foam cells. After the immediate postpartum period, cellular edema was reduced, whereas endothelial proliferation and increased mesangial cells persisted (80). Basement membrane double contours also appear to resolve rapidly after pregnancy, although these changes may persist for months in some cases (69,75). Focal segmental glomerulosclerosis occurs in some cases and is discussed on page 825.
Tubulointerstitium Tubulointerstitial changes are nonspecific. Atrophy of tubules and interstitial fibrosis parallel glomerular sclerotic changes. Proximal tubules may show protein reabsorption droplets and lipid droplets (45). Casts are present, particularly in collecting ducts, and some contain hemoglobin and stain for iron.
Blood Vessels Renal biopsies contain limited samples of large vessels, and the vascular changes observed in biopsies from preeclamptic patients are usually nonspecific. Multiple mechanisms may contribute to the lesions. Medial hypertrophy of renal and extrarenal interlobular arteries and arterioles in preeclampsia (44) may result from increased sensitivity to vasoactive substances such as angiotensin II (41) (see Clinical Findings, p. 819), which can exert trophic actions on vascular smooth muscle cells. The insudative lesions of hyalinosis of vessels
possibly are related to endothelial injury and acute changes of blood pressure in preeclampsia. Findings of arteriosclerosis and arteriolosclerosis, with intimal fibrosis and thickening and reduplication of the elastic lamina, indicate the likelihood of preexisting nephrosclerosis (see p. 821) (53,72). This lesion of intimal sclerosis of arteries or arterioles was found in approximately one third of patients with pregnancy-related hypertension and in 10 of 14 patients with known hypertension or renal disease before pregnancy (44). Vascular lesions of malignant hypertension were found in some patients with preexisting renal disease and severe toxemia resulting in death, although hypertension was not at malignant levels clinically (81). These data could be taken to indicate that more severe toxemia occurs with more severe preexisting vascular lesions. On the other hand, severe vascular lesions of apparent “malignant hypertension” in these early reports may have been related to thrombotic microangiopathy (Fig. 19.7) (79). Severe vascular lesions, including possible vasculitis and thrombosis, can also occur when antiphospholipid antibodies are present (see p. 840).
possibly are related to endothelial injury and acute changes of blood pressure in preeclampsia. Findings of arteriosclerosis and arteriolosclerosis, with intimal fibrosis and thickening and reduplication of the elastic lamina, indicate the likelihood of preexisting nephrosclerosis (see p. 821) (53,72). This lesion of intimal sclerosis of arteries or arterioles was found in approximately one third of patients with pregnancy-related hypertension and in 10 of 14 patients with known hypertension or renal disease before pregnancy (44). Vascular lesions of malignant hypertension were found in some patients with preexisting renal disease and severe toxemia resulting in death, although hypertension was not at malignant levels clinically (81). These data could be taken to indicate that more severe toxemia occurs with more severe preexisting vascular lesions. On the other hand, severe vascular lesions of apparent “malignant hypertension” in these early reports may have been related to thrombotic microangiopathy (Fig. 19.7) (79). Severe vascular lesions, including possible vasculitis and thrombosis, can also occur when antiphospholipid antibodies are present (see p. 840).
ELECTRON MICROSCOPIC FINDINGS
Early biopsy studies with electron microscopy shed light on the precise cellular abnormalities in preeclampsia (62,63,66,82,83). Swelling of glomerular endothelial cells and, to a lesser extent, of mesangial cells is a prominent feature (Figs. 19.8 and 19.9). Lysosomes may be present in both cell types. Mesangial cells and matrix are increased, and mesangial cell interposition contributes to glomerular capillary wall thickening. Vacuolization, droplets, cytoplasmic strands, lipid, dense bodies, myelin figures, and increased numbers of cell organelles can be seen in both glomerular endothelial and mesangial cells (Fig. 19.10). Glomerular epithelial cell vacuolization and swelling are also frequent (66,67,79). Foot process effacement is seen only segmentally, and it does not appear to correlate with the degree of proteinuria (38). Epithelial cell droplets were shown by immunoelectron microscopy to contain immunoglobulin complement, fibrinogen, and larger amounts of albumin (75). Lipid droplets, both extracellular and intracellular, can be present.
Glomerular subendothelial and occasional mesangial vague densities are present, depending on the timing of biopsies (Figs. 19.11 and 19.12). The material can appear fibrillar, as more or less localized dense deposits, or as granular deposits. In severe cases, fibrin tactoids may be present in the glomerular subendothelial areas, the mesangium, and, rarely, the urinary space (Fig. 19.13) (44,45). Most investigators have identified fibrillar fibrin within dense glomerular subendothelial deposits (44,45,75,78,84). In one study, these deposits decreased in size postpartum and disappeared over the first 8 days after delivery (85). Fibrin- or fibrinogen-related breakdown products, fibronectin, and matrix components localized to these areas immunohistochemically (76,84). By immunoelectron microscopy, IgM staining also localized to areas of glomerular subendothelial electron-dense deposits in thickened loops (75).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree