RENAL PATHOLOGY HISTORY
Noteworthy elucidation of the clinical and gross pathologic manifestations of kidney disease began during the 19th century with the studies of Bright, Rayer, Rokitansky, von Frerichs, and others (
1). Beginning in the second half of the 19th century and extending into the 20th century, Ellis, Fahr, and Klebs made major advances in the pathologic classification of kidney disease using light microscopy on postmortem specimens. Pioneered by Alwall, Brun, Iverson, and Kark in the 1950s, the renal biopsy allowed access to the early stages of kidney diseases and provided an opportunity to make a pathologic diagnosis that could inform clinical care. By the 1960s, the first modern renal pathologists, including Bergstrand, Churg, Germuth, Habib, McCluskey, and Spargo
were utilizing the newly available techniques of electron microscopy and immunofluorescence microscopy (IFM) to rapidly advance the understanding of kidney diseases and to refine classification and diagnosis. These advances and those that have followed are chronicled in the seven editions to date of the textbook on pathology of the kidney first published by Robert Heptinstall in 1966 (
2) and culminating, at least for now, with this seventh edition.
THE ROLE OF THE RENAL BIOPSY
The traditional approach to renal biopsy analysis is to identify the pathology by systematically examining the different histologic compartments (glomeruli, tubules, interstitium, and blood vessels). Once the site and nature of the lesions are determined, the pathologist makes a final diagnosis by integrating the histopathology with IFM and electron microscopy findings, and with clinical information including relevant laboratory data. This chapter serves as a guide to renal biopsy evaluation by focusing on each renal compartment and its pathology in turn, and it refers the reader to detailed discussions of the specific diseases in other chapters of the book.
Several factors make evaluation of renal pathology challenging, especially in renal biopsy specimens. There are a limited number of stereotypic renal responses to injury. In other words, diverse pathogenetic mechanisms may produce a similar morphologic response. As a corollary, only a few findings are pathognomonic in renal pathology such as the reaction of the Congo red stain for amyloid (see
Chapter 22), the linear staining for monoclonal immunoglobulin in glomerular and tubular basement membranes (TBMs) in monoclonal immunoglobulin deposition disease (see
Chapter 22), and the unique intramembranous dense deposits seen by electron microscopy in dense-deposit disease (DDD) (see
Chapter 9). Even the venerable Kimmelstiel-Wilson lesion of diabetic glomerulosclerosis (see
Chapter 21) and the fibrils of amyloid seen by electron microscopy (see
Chapters 22 and
23) are now subject to differential diagnoses.
The second major problem is the small size of the biopsy, although the amount of tissue that is sufficient for a specific diagnosis is influenced by the disease that is present. For example, one glomerulus with amyloid identified by light or IFM is adequate for a diagnosis of amyloidosis and one glomerulus with the pathognomonic features of DDD by electron microscopy is sufficient for diagnosis. On the other hand, failure to detect glomerular lesions in a small sample with only a few glomeruli does not allow ruling out diseases with focal glomerular lesions, such as focal segmental glomerulosclerosis (FSGS) and pauci-immune focal necrotizing glomerulonephritis. Small sample size also impairs the assessment of the overall severity, activity, and chronicity of the disease, which can be as important in prognostication and therapeutic decisions as is the specific disease diagnosis.
Another problem is that it is not always easy to identify the primary lesion because more than one compartment may be involved by the primary process, secondary processes may intervene, and major findings may be subtle. Finally, progression of many forms of renal injury toward end-stage disease results in nonspecific chronic changes that obscure the nature of the original pathologic process. As Simeon Burt Wolbach, former Chairman of Pathology at Harvard, noted, “It is often difficult to ascertain the nature of the edifice that has burnt down from a study of the ashes” [quoted in Ref. (
3)]. In spite of these problems, the pathologic interpretation of a renal biopsy specimen remains an important guide for the clinician in the diagnosis, prognosis, and therapy of renal disease.
The renal biopsy has been used to identify pathogenetic mechanisms and to establish clinicopathologic correlations between pathologic findings and clinical symptoms. The renal biopsy is frequently necessary to distinguish among diseases with similar clinical presentations. For example, the many diseases that cause the nephrotic syndrome, nephritic syndrome, and acute renal failure (ARF) have vastly different prognostic and therapeutic implications, exemplifying the importance of the renal biopsy in differential diagnosis.
Table 3.1 lists clinical manifestations of renal disease that may prompt pathologic evaluation. Traditionally, nonneoplastic renal diseases that are managed primarily by nephrologists (i.e., medical renal diseases) are diagnosed by examination of renal biopsies by renal pathologists (nephropathologists), whereas neoplastic renal diseases and diseases of the urinary tract that are managed primarily by urologists and may lead to partial or complete nephrectomy without prior biopsy are evaluated by urologic pathologists.
Table 3.2 lists most of the diseases that should be considered when evaluating native renal biopsy specimens and specifies the chapters in this book that review each category of disease.
The primary role of the renal biopsy is to provide a diagnosis and information about disease activity and chronicity that allow the clinician to make an informed prognosis and choose the optimal therapy. In some instances, a specific cause of the renal injury may be identified by pathologic examination or suggested by the pathologic findings and subsequently confirmed clinically. This may lead to elimination of the cause and resolution of the disease. Determination of the relative amount
of acute, potentially reversible injury versus irreversible scarring, which may not be apparent from the clinical findings, is equally important. In cases with advanced chronic injury, the decision not to treat lesions that are deemed to be too advanced to respond to therapy may be based on the renal biopsy findings. Furthermore, renal biopsy is the only way to recognize and describe some new renal diseases, for example, the adverse effects of new drugs. Finally, renal biopsy is required in clinical trials to ensure that the disease process and the disease severity are comparable among the study groups and to serve as a baseline for evaluating therapeutic efficacy.
TECHNICAL CONSIDERATIONS
Risks
The clinician must balance the information to be gained and its impact on patient care against the risks associated with a renal biopsy; however, renal biopsy using the spring-loaded biopsy gun with ultrasound guidance is a very safe procedure (
4). Following a biopsy procedure, microscopic hematuria occurs in about 35% of patients, but gross hematuria is seen in less than 0.5%. A perirenal hematoma is identified in approximately 65% of patients, depending upon the diligence of the search. Transfusion is required as a consequence of less than 1% of biopsies and nephrectomy in less than 0.1%. Mortality is extremely rare. To obtain optimal tissue for pathologic evaluation without increased morbidity, 14- or 16-gauge needles are recommended for renal biopsies in adults and 16- or 18-gauge needles in children younger than 8 years old (
4).
Pathologic Evaluation
Light microscopic morphology is assessed on 2- to 3-µm histologic sections stained routinely with hematoxylin and eosin, methenamine silver-periodic acid (Jones stain), Masson trichrome, and periodic acid-Schiff (PAS) (
5). Congo red and thioflavin T for amyloid may be used routinely or only when amyloidosis is suspected based on clinical or pathologic findings. An immunohistology technique (either immunofluorescence
or immunoperoxidase) to demonstrate deposits of immunoglobulins (IgG, IgM, IgA, kappa, and lambda light chains) and complement components (C3 and C1q) is required for adequate pathologic evaluation (
5). Electron microscopy is a valuable adjunct that is required for the diagnosis of some diseases, such as fibrillary glomerulonephritis and thin basement membrane nephropathy, and may reveal a diagnosis that was unsuspected after light and IFM examination, such as Fabry disease or hereditary nephritis. In addition, several special techniques are recommended, such as special immunohistochemistry for infectious pathogens (e.g., BK virus) and cell types (e.g., B lymphocytes versus T lymphocytes), and ultrastructural morphometric techniques to measure basement membrane thickness and the diameter of abnormal fibrils or microtubules. Renal biopsies should be processed only in laboratories that are proficient in the performance and interpretation of these tests (
5).
Specimen Adequacy
How much renal tissue is necessary for a pathologic diagnosis is a complex question, and the answer depends on the indication for biopsy. If the differential diagnosis includes diseases defined by immunohistology or ultrastructure, tissue must be processed for these studies as well as for light microscopy. A single glomerulus may be sufficient for the diagnosis of diffusely distributed glomerular diseases with specific pathologic features, such as amyloidosis or membranous glomerulonephritis. However, in many cases, specimen adequacy is a statistical consideration of the number of glomeruli required to answer either of two questions. First, how many glomeruli are required to exclude focal pathology? Second, what proportion of the glomeruli is involved? A discussion of these questions follows.
Diagnosis of diseases involving only a proportion of the total number of glomeruli (focal) requires the demonstration of only one abnormal glomerulus, and the relevant question is how many normal glomeruli are needed to confidently exclude focal pathology. Assuming that the disease is randomly distributed among the glomeruli, the glomeruli are independently affected, and the glomerular sample is random, the probability of finding any number of abnormal glomeruli in the renal biopsy can be represented by the binomial equation (
6). The number of abnormal glomeruli in the biopsy is a function of the sample size and the proportion of abnormal glomeruli in the kidney (
6). In a kidney with 10% glomerular involvement, a biopsy containing 10 glomeruli will have a 35% chance of having no abnormal glomeruli, but when glomerular involvement is 35%, the chance of finding no abnormal glomeruli in a biopsy with 10 glomeruli is less than 5%. Thus, a biopsy with few glomeruli cannot exclude focal disease with a low proportion of glomerular involvement, and the minimal sample needed to exclude focal disease present in fewer than 10% of the glomeruli with greater than 90% confidence is at least 20 glomeruli. Complicating the issue of adequate sampling is the possibility of segmental involvement of an individual glomerulus, defined as involvement of only a portion of the glomerular tuft area. This is particularly important in such diseases as FSGS, lupus nephritis, and pauci-immune focal crescentic glomerulonephritis, where careful serial sectioning of the biopsy increases the diagnostic yield. Most renal pathologists section each biopsy with 20 or more serial sections to maximize the likelihood of identifying such focal renal lesions.
Assignment of patients to groups (stratification) based on the proportion of abnormal glomeruli in the biopsy is a more complex problem. For example, the distribution of abnormal glomeruli found in biopsies from patients with systemic lupus erythematosus (SLE) with mild focal (less than 20%), moderate focal (20% to 50%), and diffuse (50% or more) glomerular involvement can be calculated from the binomial equation (
6). Small differences between groups (e.g., 10%) require more than 100 glomeruli to achieve statistical significance, and a minimum of 20 to 25 glomeruli is necessary to detect relatively large differences (25% to 40%).
In study design, the limitations of morphologic stratification must be appreciated or incorrectly classified patients will dilute the study outcomes. The inclusion of patients from a good prognosis group in a bad prognosis group will improve the outcome in both groups without changing the overall incidence of bad outcomes. Attention to the statistical rules will lead to results that are internally consistent within groups and reliably different between groups. A final caveat is that observations made on the even more limited samples studied by electron microscopy should be extrapolated to the whole kidney cautiously. Because of the need for integration of information gleaned from all three modalities of biopsy workup, it is important for the same pathologist to evaluate the findings by light microscopy, immunofluorescence, and electron microscopy.
Semiquantitation of Pathologic Findings
Pirani et al. (
7) pioneered the use of semiquantitative renal pathologic changes “to force the pathologist to look at all elements of renal histology” in a systematic fashion. This approach was used in the context of lupus glomerulonephritis to develop indices of disease activity and chronicity based on semiquantitative observations (
8). Additional examples of systematic approaches to semiquantitative evaluation in renal pathology are exemplified in the Banff classification for renal allograft pathology (
9,
10) and the Oxford classification for IgA nephropathy (
11,
12).
Certain quantitative and semiquantitative features should be included in every biopsy report, including the number of glomeruli; the number of glomeruli with specific lesions; the amount of mesangial matrix and an assessment of glomerular cellularity (in the mesangial, endocapillary and extracapillary zones of the glomerular corpuscle); the proportion of the biopsy occupied by interstitial inflammatory infiltrates, interstitial fibrosis, and tubular atrophy; and the distribution and intensity of immune deposits.
THE PATHOLOGIC DIAGNOSIS OF GLOMERULAR DISEASE
Glomerular diseases are the pathologic processes most often identified in renal biopsy specimens from native kidneys (
Table 3.3) (
13). The complexity and variety of glomerular diseases pose a considerable challenge for the pathologist.
Glomerular diseases have a broad variety of clinical presentations including asymptomatic hematuria, asymptomatic proteinuria, nephrotic syndrome, acute nephritis, rapidly progressive nephritis, ARF, and chronic renal failure. Pathologic evaluation of a glomerular disease by light microscopy rarely allows a definitive diagnosis. More often than not, immunohistology or electron microscopy or both are required to reach the most definitive and clinically useful diagnosis. Glomerular lesions often evolve over time, for example, as active inflammatory lesions transform into chronic sclerotic lesions. Knowledge of these dynamic transitions is important not only for diagnosis but also for prognostication, which involves assessment of the activity and chronicity of disease at the time of biopsy. Further complicating the pathologic diagnosis of glomerular diseases is the frequent concurrence of secondary pathologic changes in the tubules, interstitium, and extraglomerular vessels that may be even more conspicuous than are the primary glomerular changes.
Renal biopsy reports should use widely accepted descriptive terminology in describing glomerular pathology (
Table 3.4) as well as widely accepted diagnostic terminology (see
Tables 3.2 and
3.3). To complicate matters further, the pattern of injury observed in a renal biopsy specimen is a snapshot of a dynamic process of glomerular injury in a patient who may have different patterns of glomerular injury over time (
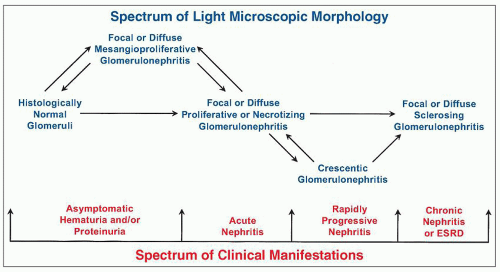
Fig. 3.1) (
14). For example, a patient with IgA nephropathy or a patient with lupus nephritis may have a mild mesangial proliferative glomerulonephritis early in the course of disease that evolves into a focal proliferative glomerulonephritis with more destructive segmental lesions, and still later progresses to a diffuse proliferative glomerulonephritis that ultimately results in chronic sclerosing glomerulonephritis.
Because each light microscopic pattern of glomerulonephritis can have many different causes with very different prognoses, recognition of the specific cause of the injury in a given specimen is as important as, if not more important than, categorizing the light microscopic phenotype. For example, consider focal glomerulonephritis caused by IgA nephropathy versus lupus nephritis versus antineutrophil cytoplasmic
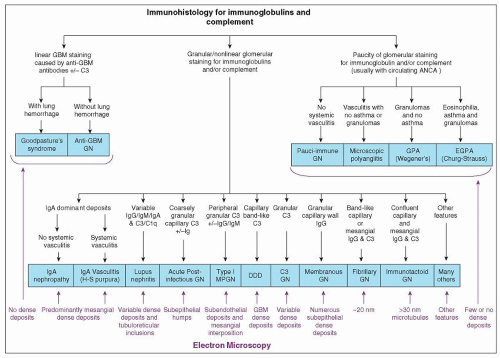
antibodies (ANCA) glomerulonephritis; each has a very different prognosis and very different lesion-specific treatment. Likewise, recognition of crescentic glomerulonephritis by light microscopy does little more than confirm the clinical impression of rapidly progressive glomerulonephritis. The more important determination is detection of the cause of the crescentic glomerulonephritis by integration of data from light microscopy, IFM electron microscopy, serology, and other laboratory and clinical observations. The algorithm in
Figure 3.2 demonstrates some of the observations by immunofluorescence and electron microscopy that are useful in resolving the differential diagnosis in a patient who has light microscopic evidence for glomerulonephritis. Bear in mind that an optimal approach to pathologic diagnosis of a glomerular disease is based not only on identifying the presence of features that are indicative of a specific disease but also on noting the absence of features that are indicative of alternative diseases.
Glomerular diseases occur not only as diseases that primarily target the kidneys but also as components of systemic diseases. For example, glomerular disease can be secondary to SLE, diabetes mellitus, amyloidosis, monoclonal immunoglobulin deposition disease, hypertension, and systemic vasculitis such as ANCA vasculitis, IgA vasculitis (Henoch-Schönlein purpura) or cryoglobulinemic vasculitis. Thus, once a distinct pattern of glomerular injury is identified, the possibility of a secondary rather than a primary process must be considered. For example, is IgA-dominant immune complex disease IgA nephropathy (a primary process) or secondary to IgA vasculitis or to staphylococcal infection? Likewise, is type I membranoproliferative glomerulonephritis (MPGN) an idiopathic (primary) process or secondary to an identifiable cause, such as cryoglobulinemia, hepatitis B infection, subacute bacterial endocarditis, monoclonal IgG, C3 glomerulopathy, and so forth? The distinction between primary and secondary disease often requires knowledgeable integration of information not only from the light, immunofluorescence, and electron microscopy observations but also from clinical and laboratory data. As much as any other anatomic pathology subspecialty, and more than most, optimal diagnosis of pathologic findings in kidney specimens requires careful correlation with clinical data. To complicate matters further, some glomerular diseases coexist, producing dual glomerulopathies. This situation is particularly applicable to common conditions. For example, membranous glomerulonephritis or pauci-immune focal crescentic glomerulonephritis may occur superimposed on diabetic nephropathy.
Thus, the pathologist should always remain open-minded to the possibility of superimposed diseases, especially when confronted with disparate pathologic changes that cannot be explained by a single disease process.
Light Microscopic Evaluation of Glomeruli
Refer to Chapter 1 for a more detailed description of glomerular structure than in the following summary. A glomerulus is composed of a tuft of capillaries supported by a mesangial core. Podocytes (visceral epithelial cells) cover the urinary surface of the capillaries and mesangium. Podocytes are continuous at the glomerular hilum with the parietal epithelium that covers Bowman capsule, which transition at the tubular pole into the epithelium of the proximal tubule. Each glomerulus has an endocapillary compartment and an extracapillary compartment separated by the GBM. The endocapillary compartment includes the endothelial cells, mesangial cells, and any leukocytes in the capillary lumens or mesangium. Normally,
in a 2- to 3-µm histologic section, there are no more than one (or rarely two) endothelial nuclei per capillary lumen and no more than three nuclei in a peripheral segment of contiguous mesangial matrix. The mesangium coalesces at the vascular pole of the glomerulus, and there may be more nuclei in the contiguous matrix at this location. The extracapillary compartment includes the visceral and parietal epithelial cells and any cells within Bowman space. Normally, there is a single layer of podocytes and parietal epithelial cells, and no cells in Bowman space. The normal glomerulus contains distinct extracellular matrix domains that comprise the GBM, the mesangial matrix, and the basement membrane of Bowman capsule.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access