Immunopathology
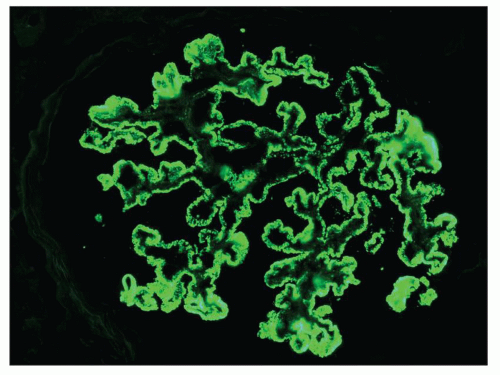
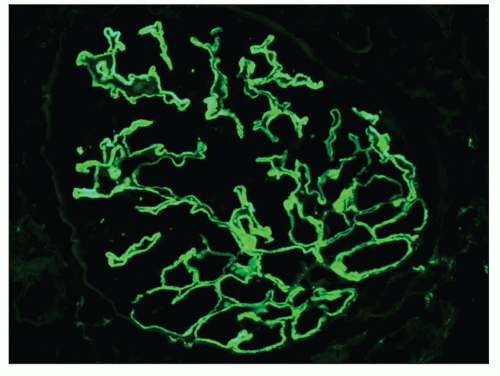
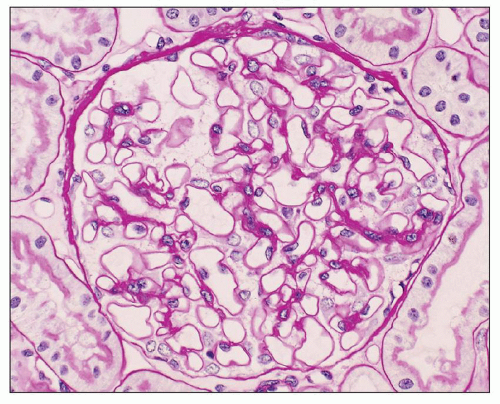
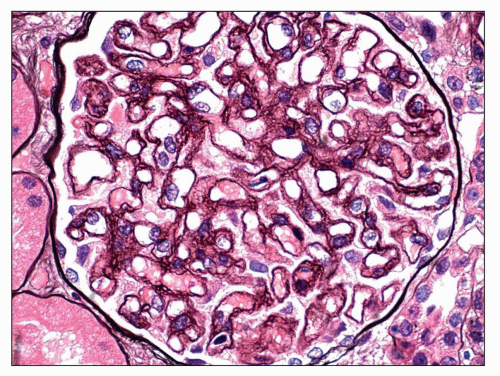
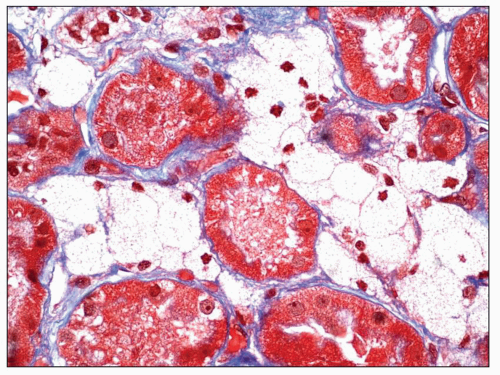
Immunopathologic studies have shown that histologic changes seen in the GBM by light and electron microscopy in MGN are due to deposits of immunoglobulin and complement components. These immune deposits are best demonstrated by immunofluorescence and correspond to the ultrastructural finding of electron-dense deposits that lie between GBM spikes. The typical immunofluorescence finding in MGN is global deposits of immune reactants that follow the contour of the GBM (
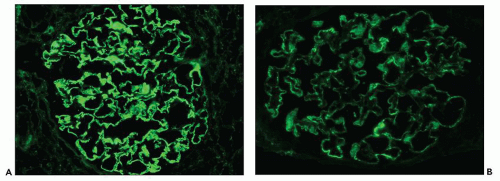
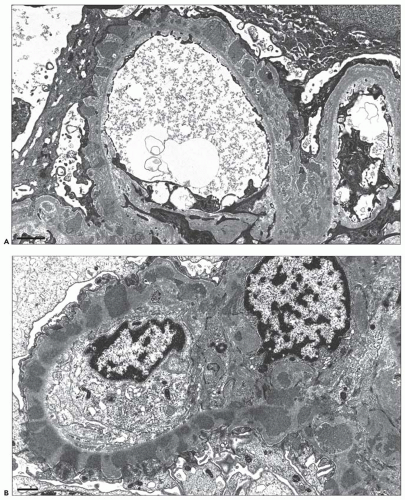
Fig. 7.14). Since the deposits lie at the subepithelial aspect of the GBM and project toward the urinary space, they typically have a granular appearance and appear relatively discrete and uniform. In contrast, a minority of cases are characterized by small, confluent deposits that produce a pseudolinear appearance (
Fig. 7.15). In rare instances, the deposits are sparse and only segmentally distributed; this mainly occurs very early in the course of disease or in a resolving phase, although rare cases of segmental MGN have been described (
52). Mesangial deposits are present in less than 10% of cases of primary MGN (
53) but are important to identify as their presence favors a secondary form of disease. Mesangial deposits can be difficult to differentiate by immunofluorescence from subepithelial deposits that follow the GBM reflection around mesangial areas (
33).
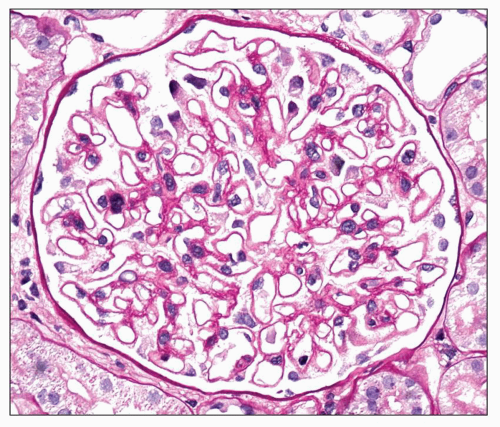
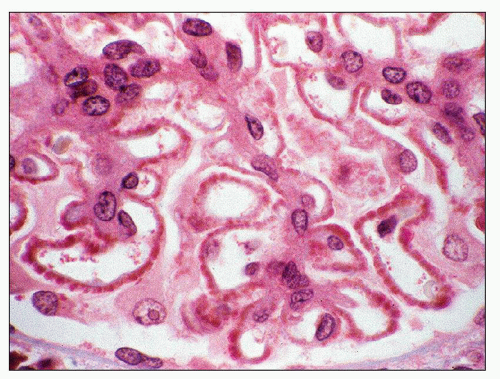
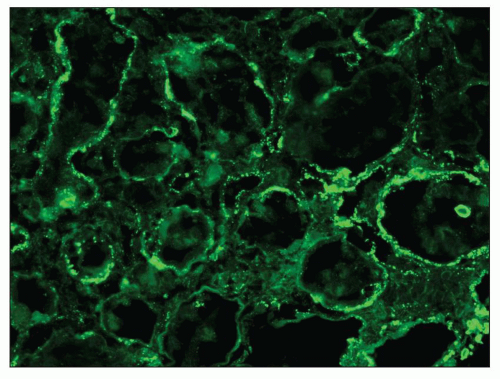
The principal and invariable component of the subepithelial immune deposits in primary MGN is IgG (
Fig. 7.16), with a composition that includes both kappa and lambda light chains. Staining for complement component C3 is also usually present, seen in 85% of cases in one large series (
33). Staining for IgM, IgA, and C1q is present in 47%, 16%, and 23% of cases, respectively (
33). The intensity of staining for IgG is greatest in virtually all cases, and staining for IgM, IgA, and C1q is typically of no more than 1+ intensity (scale +/-, 1+ to 3+). The intensity of staining for C3 is more variable and typically less than that of IgG; few cases may have equivalent staining to IgG. There are rare cases of MGN in which the deposits are monoclonal and exhibit light chain restriction and IgG subclass restriction, most commonly IgG1-kappa (
54,
55).
Not surprisingly, these cases can be associated with hematologic malignancy (
54,
55).
Immunopathologic findings are often helpful in distinguishing primary MGN from membranous LN and, to a lesser extent, other secondary forms of disease (
33). In comparison to primary MGN, membranous LN is characterized by a greater prevalence of positive staining for IgM (62% vs. 47%), IgA (38% vs. 16%), C3 (95% vs. 85%), C1q (67% vs. 23%), and, in particular, intense, ≥2+ staining for C1q (67% vs. 11%) (
33). Combined staining for IgG, IgM, and IgA carries a sensitivity of 29% and a specificity of 87% for the diagnosis of membranous LN versus primary MGN, while intense, ≥2+ staining for C1q has a sensitivity of 67% and a specificity of 88% (
33). The classic description and hallmark of membranous LN is “full house” staining for immunoglobulins and complement, an expression derived from the game of poker, which refers to the presence of
three of a kind (three immunoglobulins: IgG, IgM, and IgA) and
two of a kind (complement components: C3 and C1q). Importantly, many of these findings can also be seen in other secondary forms of MGN. For instance, staining for IgG, IgM, IgA, C3, and C1 are each individually present in greater than 50% of biopsies with MGN secondary to HBV infection (
32).
There are four distinct subclasses of IgG (IgG1, IgG2, IgG3, and IgG4), which nonetheless exhibit 95% homology with one another (
56). The numbering of the subclasses reflects their relative prevalence in human serum, with IgG1 representing 66% of circulating IgG and IgG4 comprising only 4%. The small differences in amino acid sequence have significant functional consequences. For instance, IgG3 has the greatest ability to activate complement, the highest affinity for Fc receptors on phagocytic cells, and a significantly shorter serum half-life than the remaining three subclasses (7 vs. 21 days) (
56). IgG1 also effectively fixes complement and binds to phagocytic cells. IgG4, the least prevalent subclass of IgG, is the only subclass that does not significantly activate complement (
56). IgG4 has the unique property of weak disulfide bonds between the two heavy chains, allowing for dissociation into ½ IgG4 molecules composed of a single light and heavy chain and then reassembly with alternative ½ IgG4 molecules to form a heterobivalent IgG4 with two distinct specificities (
57). Because the resultant IgG is bispecific, cross-linking of antigens is extremely unlikely, leading to the belief that IgG4 may serve to down-regulate the immune response (
56,
57). IgG4 is thought to play an important role in multiple disease processes, including IgG4RD and, more notably, MGN.
The predominant IgG subclass in the subepithelial deposits of primary MGN is IgG4 (
58,
59,
60,
61,
62,
63) (
Table 7.2). While this is a well-established finding, the reasons remain somewhat unclear. IgG4 is not significantly increased in the serum of patients with MGN (
61,
62,
63). The IgG4 immune response is thought to require prolonged antigenic exposure (
57) and involves T-helper (Th)2 cell-mediated B-cell stimulation (
64). It is possible that the IgG4 in MGN represents a protective response against cross-linking of antigens by other subtypes of
IgG, for instance IgG1 (
64). This possibility is supported by the observation that C3 is present in the subepithelial deposits in 85% of cases of MGN (
33), a finding that cannot be explained by the presence of IgG4 alone, unless it is capable of activating complement through the lectin pathway (
64,
65). A recent study reconfirmed the dominance of IgG4 in primary MGN but noted that IgG1 predominated in early, stage I disease, suggesting that an IgG subclass switch occurs early in the course of primary MGN (
66).
While IgG4 is the predominant subclass in the subepithelial deposits of primary MGN, IgG1 is also present in the majority of cases and is often accompanied by small amounts of IgG2 and IgG3 (
58,
59,
60,
61,
62,
63) (see
Table 7.2). Not surprisingly, IgG4 is also the dominant subclass when MGN recurs in the allograft (
67). In contrast, IgG1 tends to predominate in MGN secondary to malignancy (
58) or mercury exposure (
68) and in de novo MGN in the renal allograft (
63). In membranous LN, IgG1, IgG2, or IgG3 may predominate and all three typically stain more intensely than IgG4, although IgG4 is present in the majority of cases (
59,
60,
62,
63,
69). Staining for IgG subtypes has been proposed as a modality to differentiate primary and secondary forms of MGN. In our experience, this approach is of somewhat limited utility due to the extensive overlap in staining patterns seen in
Table 7.1 as well as the significant subjectivity in grading of positivity. Nonetheless, this approach is likely to be helpful when the main diagnostic considerations are primary MGN and membranous LN. Fortunately, staining for the PLA
2R has emerged as a far more effective strategy to differentiate primary and secondary forms of disease (
70,
71,
72,
73).
Given that primary MGN is causally linked to the development of anti-PLA
2R antibodies and that renal expression of the PLA
2R is limited to podocytes, it is not surprising that extraglomerular deposits are extremely uncommon in primary MGN. For instance, in two series involving 142 and 26 patients with primary MGN, extraglomerular deposits were not identified in any patient (
33,
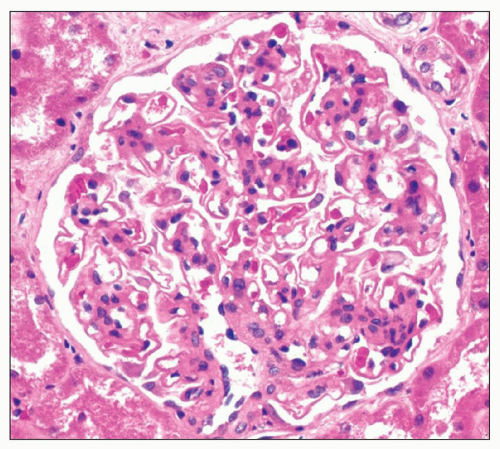
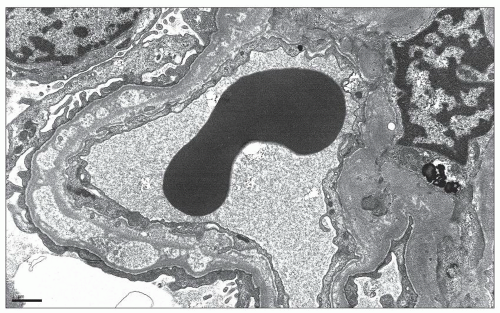
74). In contrast, the finding of extraglomerular deposits favors a secondary form of the disease. In particular, extraglomerular deposits involving tubular basement membranes (TBMs) are present in 30% to 50% of cases of membranous LN (
33) and are often accompanied by interstitial and vessel wall deposits (
Fig. 7.17). We have reported a series of three patients with MGN and prominent Bowman capsular and TBM deposits in which a secondary etiology was not identified (
75).
There are reports of children with anti-TBM nephritis and concurrent MGN. This entity is mainly seen in male children between the age of 2 months and 10 years (
76,
77), and an X-linked pattern of inheritance has been reported in two families with four affected male offspring (
78). Clinical presentation typically includes proteinuria, renal insufficiency, and Fanconi syndrome, and serum studies may document the presence of anti-TBM antibodies. In additional to findings of MGN, pathologic evaluation reveals tubulointerstitial nephritis (TIN) with linear staining of TBMs for IgG, kappa, and lambda by immunofluorescence, and EM often demonstrates electron-dense deposits within TBMs. Indirect immunofluorescence applying the patient’s serum to normal kidney reveals linear staining of TBMs and may be used to confirm the diagnosis. The anti-TBM antibodies seen in this condition cross react with the TBM of multiple animal species (
79). By Western blot, the antibodies react with a 58-kDa noncollagenous glycoprotein component of TBMs (
79) that exhibits 30% homology with human preprocathepsin B (
80) and interacts directly with type IV collagen and laminin, suggesting a role in tubular epithelial cell adhesion to TBMs (
81). The 58-kDa
anti-TIN antigen has not been shown to be expressed in podocytes, although possible cross-reactivity of anti-TBM antibody with a podocyte antigen has been proposed (
77). Prognosis for this condition is poor, with frequent progression to end-stage renal disease (ESRD).
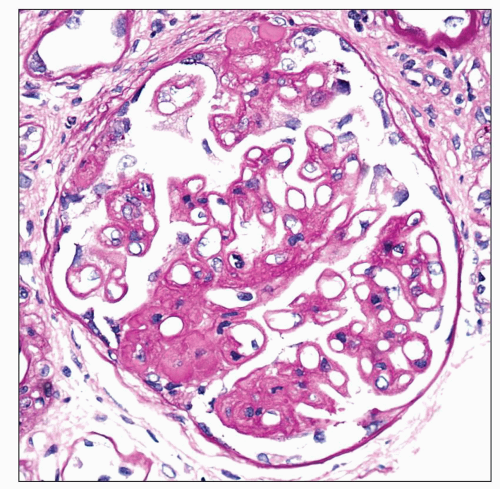
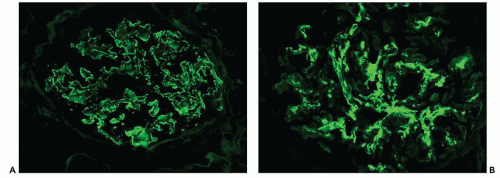
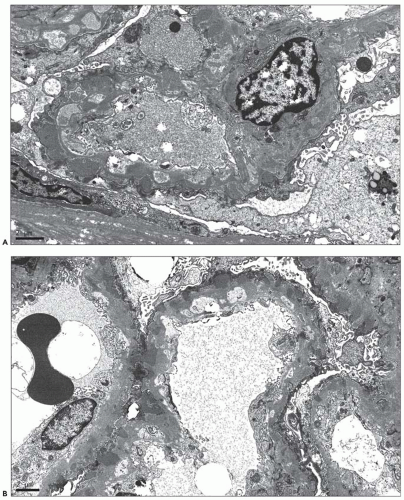
A small subset of patients with MGN exhibit mesangial deposits that, in contrast to the subepithelial deposits, stain intensely for IgA with minimal to absent staining for IgG. These cases represent MGN with coexistent mesangial IgAN (
34,
35,
82) (
Fig. 7.18). Patients with combined MGN and IgAN are typically adults who present with proteinuria and hematuria. Given the separate and distinctive pathogenesis of these two disease entities and rarity of this combination, a unified mechanism of disease is unlikely. Combined MGN and IgAN appear to be common in patients with hepatitis B infection, in patients with Asian ethnicity, and in the allograft, where IgAN recurs in a large percentage of cases and MGN is among the most frequent de novo form of glomerular disease (
34).
Electron Microscopic Findings
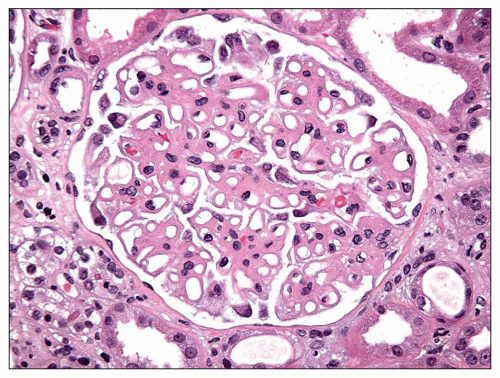
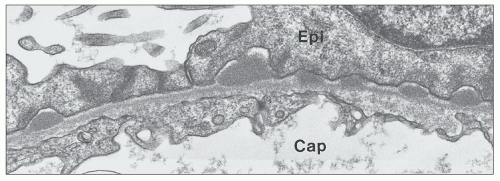
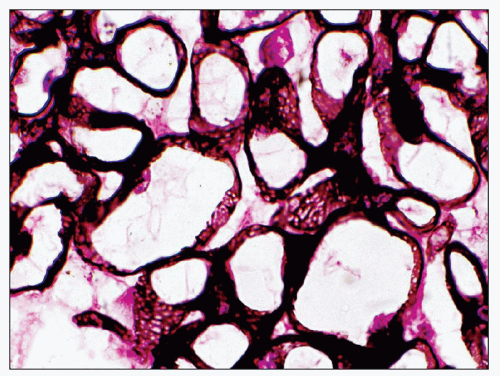
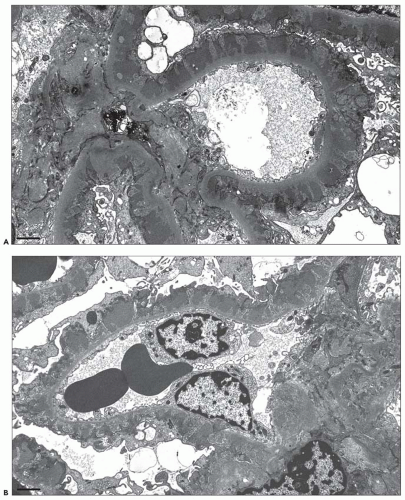
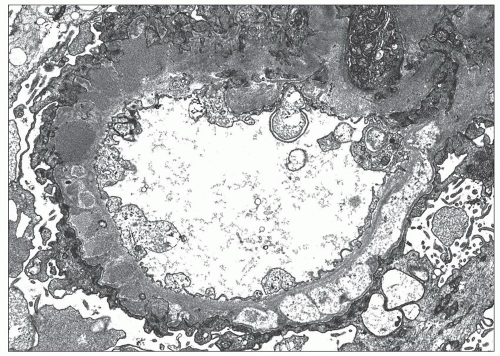
EM plays a central role in establishing the diagnosis of MGN, as it demonstrates both the immune complex deposits identified by immunofluorescence and the GBM “spikes” seen best by light microscopy. EM also is needed to accurately determine the stage of MGN.
MGN is defined by the presence of
subepithelial deposits that form on the outer aspect of the GBM, beneath the podocyte. The deposits appear to sit on the GBM and are accompanied by a spectrum of GBM changes ranging from intervening projections of the extracellular matrix (“spikes”) to areas where the GBM projections surround and encase the deposits, creating the appearance of a newly formed, overlying “neomembrane.” The deposits can range from electron dense to pale and electron lucent, consistent with focal resorption. In the absence of neomembrane formation, the deposits are in direct contact with and may indent the cytoplasm of the overlying podocytes. Podocytes exhibit a spectrum of reactive changes including condensation of actin filaments, increased cytoplasmic organellar content, lipid and protein resorption droplets, microvillous transformation, and foot process effacement. Animal models have shown that at a molecular level, podocytes exhibit loss of nephrin expression, dissolution of the actin cytoskeleton, and loss of slit diaphragm integrity (reviewed in (
83)).
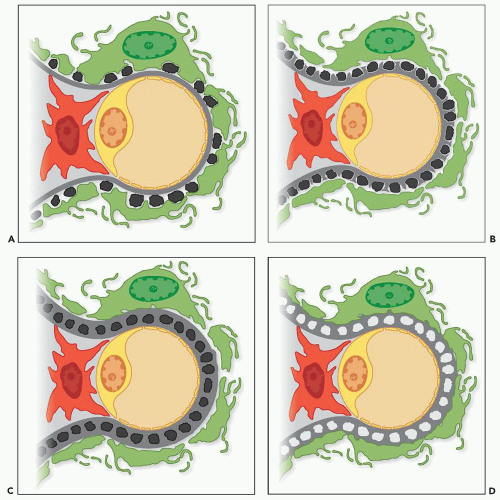
The range of ultrastructural findings seen in MGN led Ehrenreich and Churg (
27) in 1968 to propose a morphologic classification that describes a pathologic sequence of subepithelial immune deposit formation, reactive GBM changes, and, in some cases, subsequent resorption of deposits and GBM repair (
Fig. 7.19). This classic publication describes four sequential stages of MGN that provide general information about the relative age of the glomerular lesions.
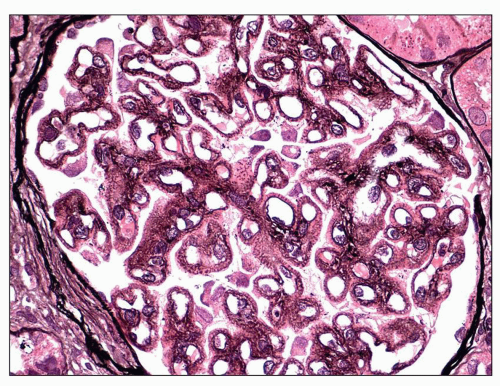
Stage I MGN is characterized by subepithelial electron-dense deposits that are typically small, sparsely distributed, and by definition devoid of significant intervening GBM spike formation, although small depressions in the GBM may be noted (
27) (
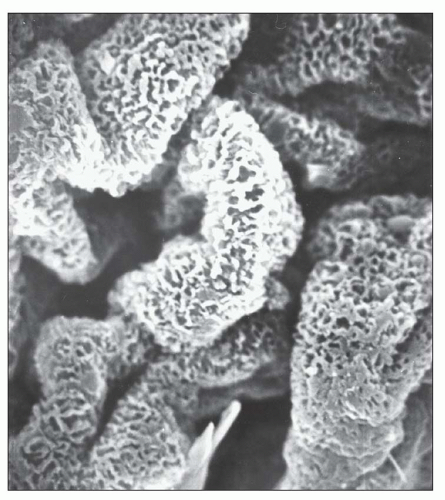
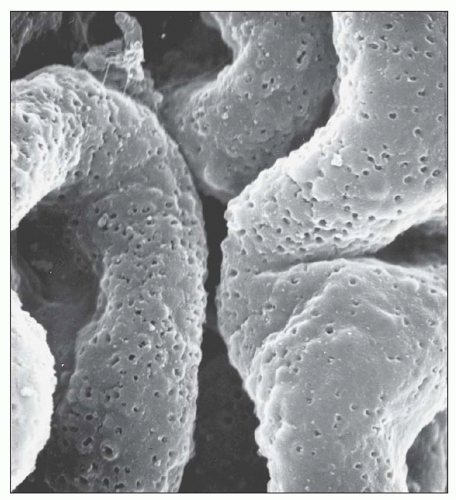
Fig. 7.20). In the majority of cases, no significant abnormalities are identified by light microscopy with the exception of cases in which focal depressions of the GBM may be noted with the JMS stain or fuchsinophilic deposits are apparent with the trichrome stain. The diagnosis of stage 1 MGN is easily established by immunofluorescence and EM. Given that stage 1 represents the earliest changes of MGN, it is not surprising that, unlike the other stages, the deposits may be only segmentally distributed. Scanning EM of acellular glomeruli shows shallow depressions on the epithelial side of the GBM (
84) (
Fig. 7.21).
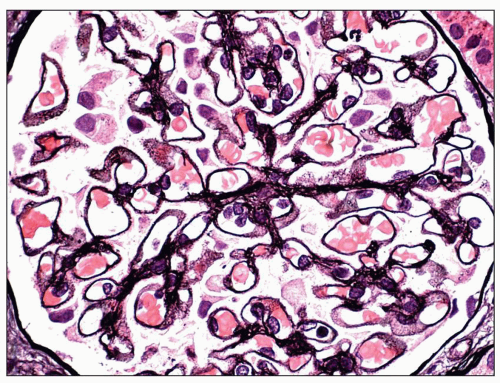
Stage II MGN is characterized by subepithelial electron-dense deposits that appear larger than the deposits in stage 1 and by definition are surrounded by intervening projections of the GBM, referred to as GBM “spikes” (
27) (
Fig. 7.22). By light microscopy, there is global thickening of the GBM. GBM spikes are best visualized with the JMS stain but are also apparent with the PAS stain. Similar to stage 1, the
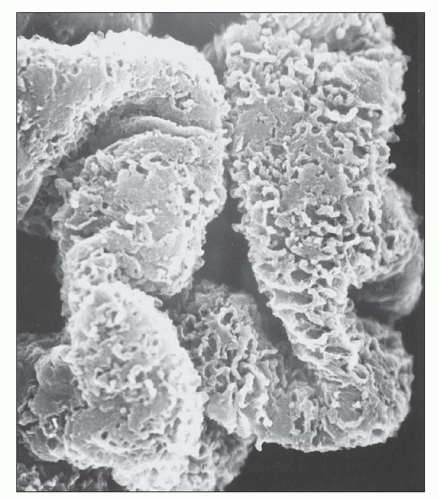
deposits typically stain intensely by immunofluorescence. The three-dimensional appearance of the deposits can be partially appreciated by scanning EM of acellular glomeruli, where the elongated deposits are surrounded by a complex anastomosing network of basement membrane projections (
84) (
Fig. 7.23).
In
stage III MGN, a new layer of GBM is laid down over the subepithelial deposits and connects the GBM spikes (
27). As a result, the deposits appear to sink into the GBM (
85) and are often described as having an intramembranous appearance, lying beneath a neomembrane (
Fig. 7.24). In stage III, the deposits can be electron dense, similar to stages I and II, or may exhibit diminished electron density, indicating that they are undergoing resorption and incorporation into the GBM. The intensity of immunofluorescence staining is variable in stage III, as less intense staining is seen in cases in which the
deposits have a more electron-lucent appearance. By light microscopy, GBM thickening is most pronounced in stage III, and the JMS stain typically shows a complex, vacuolated GBM, while the deposits usually are not visible with the trichrome stain. Although the staining intensity may be reduced, the deposits are usually demonstrable by immunofluorescence. The findings of pure stage III MGN suggest a single generation of deposits, presumably formed over a similar time period. In contrast, most cases of stage III MGN have a mixed appearance with fresh deposits layered over older intramembranous deposits, suggesting a dynamic process of ongoing deposit formation, incorporation, and resorption. In such cases, it may be possible to assign a dominant stage based on the major pattern present. In many cases, two stages coexist, making it most appropriate to designate both stages (such as mixed stage II to III) (
Fig. 7.25). By scanning EM, the complex network of GBM projections seen in stage II MGN is replaced by bridged segments of the neomembrane in stage III that have a relatively smooth appearance (
84) (
Fig. 7.26).
In
stage IV MGN, the subepithelial deposits lie within the GBM and no longer have an electron-dense appearance (
27). In contrast, the deposits either are electron lucent or have a similar electron density to the surrounding GBM owing to remodeling of the extracellular matrix. These findings are irregularly distributed, leading to a GBM that ranges from markedly expanded to more normal in thickness. In some cases, the main finding is a layer of intramembranous vaguely electron-lucent material that may more closely approximate the subendothelial than the subepithelial aspect of the GBM (
Fig. 7.27). By light microscopy, the GBM appears thickened and GBM vacuolations may be appreciated with the JMS and PAS stains. Ehrenreich and Churg also described a stage V of MGN characterized by capillary collapse, sclerosis, capsular adhesions, and crescents. In current clinical practice, these stage V changes, which unlike the previous stages are not based mainly on EM findings, are largely viewed as part of MGN stage IV. In stage IV (and stage V), immunofluorescence staining for IgG is typically of low intensity and can be absent, making the diagnosis of MGN difficult. In these instances, the finding of focal areas more typical of MGN II or III is of great assistance in establishing the diagnosis.
Although it represents a significant advance in our understanding of the morphogenesis of MGN, the Ehrenreich and Churg (
27) classification of MGN into four stages of disease
has certain limitations. First, the staging system provides insight into the evolution of disease but should not imply that all cases necessarily progress from stage I to stage IV. Second, it is often difficult to classify a case of MGN into a discrete stage because multiple stages may overlap, particularly when a fresh generation of deposits develops over an older layer of intramembranous deposits (
85,
86). Third, the morphologic stages do not correlate well with the level of proteinuria, renal function, or outcome. Fourth, progression from one stage to another can be associated with clinical improvement or worsening of disease. For example, transition from stage II to III to IV may be seen in the setting of resolution of clinical disease, while a patient may progress to advanced renal failure with biopsy findings of only stage II to III. Finally, clinical remission may be associated with no change in the appearance of the deposits, evolution to stage IV, or, less commonly, complete resolution with restoration of a normal glomerular architecture (
27,
87,
88,
89,
90).
Subepithelial deposit formation in MGN is a dynamic and potentially reversible process. The limitations of the Ehrenreich and Churg classification stem from the fact that the four stages are likely accurate for a single generation of deposits but do not address the complexity of ongoing and simultaneous deposit formation, incorporation into the GBM, and in many cases, resorption of the deposits, leading to GBM remodeling and potential healing. The clinical and pathologic resolution of disease is likely most dependent upon diminishing the rate of antibody production and deposit formation. Stated differently, outcomes are likely dependent on creating an imbalance such that the rate of healing and remodeling outpaces the rate of deposit formation. Along these lines, it has been suggested that the degree of thickening of the GBM indicates a more prolonged disease course (
85), and the finding of homogeneous, synchronous deposits (i.e., deposits of a single stage) has been shown to have a significantly better prognosis than heterogeneous deposits (i.e., multiples phases of deposits) (
91) (
Fig. 7.28). Along the same lines, another method to prognosticate in MGN may be to measure the percentage of deposits that have an electron-lucent appearance, indicating at least partial resorption; in our experience, cases with a preponderance of electron-lucent deposits tend to have lesser degrees of proteinuria and are often in a state of at least partial remission. Future studies may be helpful to address this issue.
While the subepithelial deposits are globally distributed in the majority of cases of MGN, there are multiple reports of
segmental MGN (
52,
92,
93,
94,
95) (
Fig. 7.29). The term “segmental MGN” should be reserved for cases with a segmental distribution of the deposits by light microscopy, immunofluorescence, and EM. The pathogenetic significance of segmental MGN, as compared to the more commonly encountered global lesion, is not well understood. It is likely that some of the cases, in particular those that exhibit stage I changes, represent an early manifestation of the usual form of (global) MGN, and it has been proposed that other cases may represent a resolving phase of MGN (
92). Obana et al. (
93) compared 27 children with global MGN to 11 children with segmental MGN and found that the segmental lesion was associated with a higher incidence of C1q positivity by immunofluorescence and mesangial deposits by EM but a similar clinical presentation and outcome. Interestingly, only 3 of the 11 patients with segmental MGN had stage I changes, and 2 patients underwent repeat biopsy at 3 years and were found again to have segmental deposits, arguing in favor of segmental MGN being a distinctive variant of MGN rather than an early manifestation of global MGN (
93). Based on the limited information available, segmental MGN is most commonly reported in children (
92,
93,
94). There are reports of segmental MGN in adults and, in most cases, the findings are superimposed on another pattern of glomerular injury. Bertani et al. (
52) described four adult cases of segmental MGN, all of which coexisted with other glomerular lesions including minimal change disease (two patients), diabetic glomerulosclerosis, and hereditary nephritis. In a recent report on MGN with antineutrophil cytoplasmic antibodies (ANCA)-associated necrotizing and crescentic glomerulonephritis, the membranous changes were only segmentally distributed in 6 of 13 cases (
95).
The electron-dense deposits in primary MGN are usually amorphous and finely granular, with notable exceptions (
96,
97,
98,
99,
100,
101). Kowalewska et al. (
97) described 14 patients with a variant of MGN in which the subepithelial deposits exhibit a unique, microspherical substructure with a mean diameter of 85 nm (
Fig. 7.30). Although these structures are the approximate size of nuclear pores, the authors were unable to identify nuclear pore antigens as a source for the spherules. Clinical presentation and outcomes were similar to other cases of primary MGN with the exception of associations with autoimmune diseases (SLE or Sjögren syndrome) in two patients. There are
reports of cases in which light microscopy and immunofluorescence strongly suggest the diagnosis of MGN, but EM reveals deposits that exhibit a fibrillar or microtubular substructure (
96,
98,
99,
100,
101). Based on their ultrastructural appearance, these cases are best considered as examples of fibrillary glomerulonephritis and immunotactoid glomerulopathy, respectively, and should not be considered as variants of MGN (
96,
98,
99,
100,
101). Podocyte infolding glomerulopathy is a recently described entity that resembles MGN by light microscopy and may or may not exhibit granular positivity for IgG by immunofluorescence (
102,
103,
104). In contrast to MGN, EM reveals podocyte infolding with microspherical and microtubular structures within the GBM. The majority of cases of protein infolding glomerulopathy have occurred in Japan, and many of the patients have evidence of SLE (
102,
103,
104). Although the morphogenesis of this lesion is unknown, the irregular outer contours of the GBM suggest that podocyte cytoplasmic fragments and cell membranes may become trapped in the course of deposit resorption and matrix remodeling. Finally, organized electron-dense deposits can be seen in membranous LN, and occasional cases of apparently primary MGN have unexplained organized deposits.
Mesangial electron-dense deposits are uncommon in primary MGN (
33,
105) and, when present, a secondary form of MGN should be carefully excluded (
Fig. 7.31). Follow-up is necessary in MGN patients with mesangial deposits because SLE may manifest after a latent period of several years (
14,
33). Shearn et al. (
105) found mesangial deposits in 9 of 107 cases (8.5%) of MGN in which SLE was not detected during a mean follow-up period of 9.8 years. In a series of 53 children with primary MGN followed for a mean of 53 months who did not have evidence of SLE or HBV, the Southwest Pediatric Nephrology Study Group (SPNSG) (
14) reported an incidence of mesangial deposits of 31%, which is significantly higher than has been reported in adults. Jennette et al. (
33) found mesangial deposits in 11% of 142 patients with primary MGN compared with 96% of 28 patients with membranous LN. Lai et al. (
32) found mesangial deposits in 20 of 26 (76.9%) patients with membranous LN and 11 of 22 (50%) patients with MGN secondary to HBV infection. In addition to SLE and HBV infection, mesangial deposits have been described rarely in patients with MGN related to metastatic carcinoma and following treatment with penicillamine (
36).
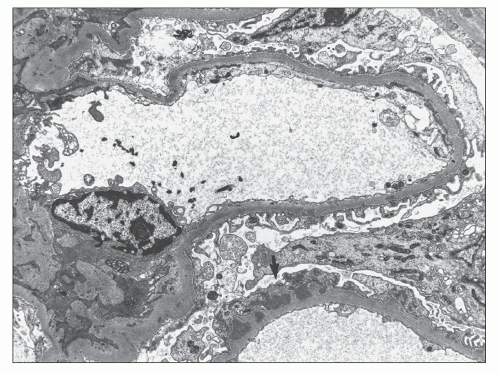
Subendothelial and extraglomerular deposits are also rarely encountered in the setting of MGN and, when present, a secondary form of disease is strongly favored (
Fig. 7.32). Jennette et al. (
33) described subendothelial deposits in 61% of 28 patients with membranous LN but only 3% of 142 patients with primary MGN. Similarly, TBM deposits were identified in 32% of 28 patients with membranous LN versus none of the 142 patients with primary MGN (
33) (see
Fig. 7.17). The SPNSG found subendothelial deposits in 9 of 9 children with membranous LN, as compared to 13% of 45 children with primary MGN (
14). Lai et al. (
32) found subendothelial deposits in 21 of 26 (80.8%) patients with membranous LN and 15 of 22 (68.2%) patients with MGN secondary to HBV infection. Another ultrastructural finding that favors a secondary form of MGN is endothelial tubuloreticular inclusions (
Fig. 7.33), which are identified in 32% to 73% of cases of membranous LN (
19,
89) and 13.4% of cases of MGN secondary to HBV infection (
32) but were absent in all 142 patients with primary MGN in at least one series (
33).
Combined Membranous and Crescentic GN
The subepithelial deposits and associated GBM changes that characterize MGN do not typically result in GBM rupture or crescent formation. Nonetheless, crescents are encountered in a small percentage of cases of MGN and, when present, should raise the possibility of concurrent SLE, anti-GBM disease, or systemic vasculitis and should lead to a serologic workup that includes testing for antinuclear antibodies, anti-GBM antibodies, and ANCA (
Fig. 7.34). The topic of mixed membranous and proliferative/crescentic forms of LN is covered in Chapter 14.
The first case of combined membranous and crescentic GN due to the presence of anti-GBM antibodies was reported by Klassen et al. in 1974 (
106). In 1984, Pettersson et al. (
107) reviewed 11 similar cases and observed that the relationship between MGN and anti-GBM disease fell into three temporal patterns: anti-GBM disease followed by MGN, MGN followed by anti-GBM disease, and patients in whom MGN and anti-GBM disease were simultaneously detected. The topic of MGN and anti-GBM disease has been the subject of more recent reviews (
108,
109). At present, there are 28 reports of this dual glomerulopathy (
108) including 5 patients in whom MGN preceded the development of anti-GBM disease, 5 patients who initially presented with anti-GBM disease and subsequently developed MGN, and 18 patients who were simultaneously diagnosed with both entities. Based upon scant data, patients with anti-GBM disease before MGN tend to be younger and have a better prognosis than those with MGN before anti-GBM disease (
108). Among the 28 patients, clinical recovery was noted in 11 patients, including 4 with anti-GBM disease before MGN and 7 who were simultaneously diagnosed.
The pathogenesis of MGN with anti-GBM disease is obscure. It has been proposed that MGN may damaged the GBM and expose cryptic epitopes that incite anti-GBM antibody formation (
106), and the converse mechanism may be operative in cases of MGN that follow Goodpasture syndrome, in which the anti-GBM antibodies may incite an immune response to a podocyte or planted antigen. Interestingly, exposure to mercuric chloride in Brown Norway rats leads to a biphasic immune response with initial development of anti-GBM antibodies and subsequent development of proteinuria and MGN (
110). The diagnosis of MGN with anti-GBM disease may be challenging because the granular subepithelial deposits of MGN may obscure the linear anti-GBM antibody staining. As a result, testing for anti-GBM antibodies should be performed in all patients with MGN and significant crescent formation.
The first case of membranous and crescentic GN related to ANCA seropositivity was reported in 1993 in a patient with Wegener granulomatosis (
111). In 1997, Tse et al. (
112) reported 10 cases of “vasculitic GN” in patients with MGN. All 10 biopsies revealed MGN with cellular and/or fibrous crescents. ANCA serologies were positive in only four of the nine patients who were tested, raising the question of whether the crescents can be attributed to a vasculitic mechanism in the absence of ANCA seropositivity. MGN and “vasculitic GN” were simultaneously diagnosed in nine patients, while one had MGN with a subsequent crescentic transformation (
112). The authors of this study reviewed the relevant literature at the time and found 10 similar cases, although only one had documented ANCA seropositivity (
111).
In 2009, Nasr et al. (
95) described 14 cases of MGN with ANCA-associated necrotizing and crescentic GN.
ANCA serology was positive by indirect immunofluorescence or ELISA in all cases and was identified as P-ANCA with specificity for myeloperoxidase (MPO) in most cases. The cohort consisted with 8 men and 6 women with a mean age of 59 years. The mean serum creatinine was 4.4 mg/dL, and 12 of 14 patients presented with acute kidney injury (AKI). The mean 24-hour urine protein was 6.5 g/d, all of the patients had evidence of hematuria, and six had extrarenal manifestations of vasculitis (
95). The diagnosis of MGN and ANCA-associated GN were established simultaneously in 13 patients, while one had been previously diagnosed with MGN. On pathologic evaluation, the findings of MGN were typically stage I or II and were only segmentally distributed in six cases. Twelve of thirteen patients with available follow-up were treated with steroids and cyclophosphamide; among these patients, 7 had stabilization or improvement in renal function, 1 had worsening renal function, and 4 progressed to ESRD (
95).
Rare cases of MGN with crescents are seen in the absence of ANA, ANCA, or anti-GBM antibody seropositivity. Crescentic transformation should be considered in the differential diagnosis of the patient with MGN who develops a rapid decline in renal function with a nephritic urine sediment.