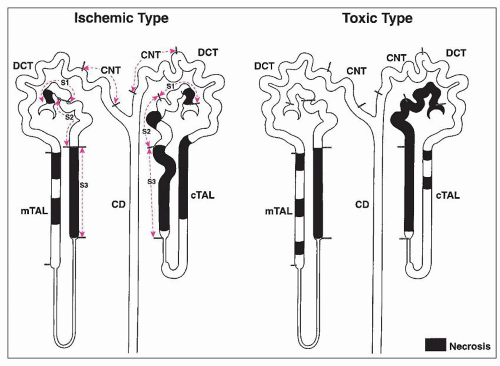
ANTIBIOTICS
Antiviral Agents The nephrotoxicity of antiviral drugs has been reviewed (
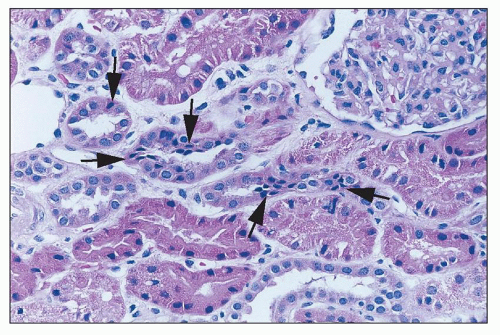
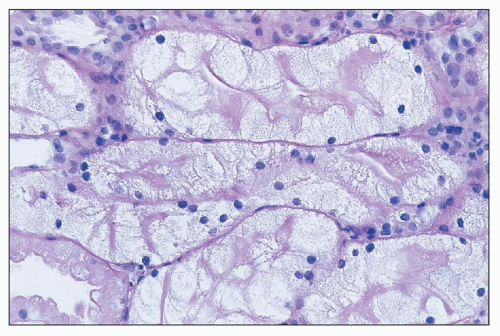
44,
45,
46,
47,
48). Acyclovir, an early nucleoside analog, was reported to be associated with renal dysfunction, and there is a significant incidence of renal dysfunction with newer agents as well. Tubular injury may lead to proximal tubulopathy and even Fanconi-like syndrome (cidofovir, tenofovir, foscarnet), distal tubular acidosis (foscarnet) and nephrogenic diabetes insipidus (foscarnet, tenofovir). The nucleoside reverse transcriptase inhibitors didanosine, stavudine, and lamivudine have rarely been associated with renal tubular dysfunction with acidosis and hypophosphatemia. Adefovir has been reported to produce proximal tubulopathy in up to 50% of patients at high doses (
49). A variety of these agents have been associated with renal failure, including acyclovir (
50,
51), foscarnet (
52), ganciclovir (
53), cidofovir (
54), indinavir (
55,
56), tenofovir (
57,
58), ritonavir (
59), and adefovir (
49). The widely used agent tenofovir has a rate of renal failure of less than 1% in those without preexisting disease (
58), though renal failure is more frequent in those with preexisting disease. Risk factors for toxicity include baseline renal dysfunction, low CD4 counts, low body weight, and concomitant use of lopinavir or didanosine (
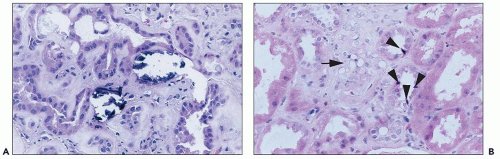
47). Some agents are associated with crystalluria and/or nephrolithiasis (acyclovir, ganciclovir, indinavir, and much less commonly with the newer protease inhibitors atazanavir, saquinavir, nelfinavir, and lopinavir-ritonavir), and flank or abdominal pain may occur. Incidence may be up to 20% with antiretroviral regimens including indinavir, and more frequent when indinavir is pharmacologically boosted with ritonavir. Crystals are needle shaped and birefringent under polarized light and can be seen in voided urine. Proteinuria has also been described with cidofovir, more commonly than ARF.
Toxicity is dose dependent, and volume depletion may predispose to toxicity. For some agents such as tenofovir, toxicity depends on accumulation of drug in proximal tubular cells and may take weeks to months for injury to be detected (
44). Renal failure often resolves rapidly when these drugs are discontinued, and the patient may be rechallenged with a lower dose of the drug without development of renal dysfunction. However, occasional cases of chronic renal failure have been reported in patients receiving cidofovir (
60), indinavir (
48), and tenofovir (
57,
61). Renal tubular functional defects may persist as well. Hydration to maintain diuresis may prevent renal toxicity, especially in those agents causing crystalluria. With acyclovir, toxicity has been described frequently with intravenous administration, but cases have been reported with oral administration as well. Combination with other nephrotoxic agents may enhance toxicity (
57). Older age and preexisting renal failure are risk factors for ARF in patients receiving acyclovir. Preexisting renal impairment, common in the HIV population, is also a risk factor for ARF induced by tenofovir, cidofovir, foscarnet, indinavir, interferon, and ritonavir, and dosage adjustment is required. Because of renal toxicity, adefovir, cidofovir, and indinavir are not approved/recommended for primary antiretroviral therapy.
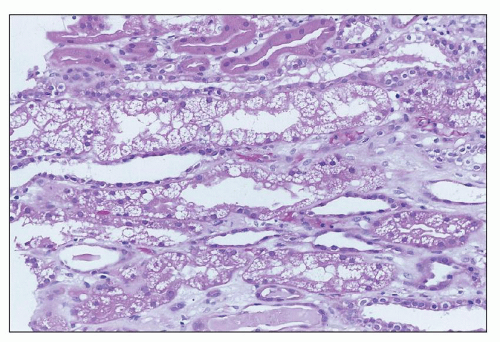
Aminoglycosides Aminoglycoside antibiotics have long been recognized as nephrotoxic and ototoxic. They continue to be used, however, because of their efficacy in treating gram-negative infections. The incidence of ARF in patients treated with gentamicin is about 20% (
62). The nephrotoxicity of the various aminoglycosides is greatest in those with the largest number of free amino groups (
63). Streptomycin, the least toxic, has two amino groups: those with intermediate toxicity, such as gentamicin, tobramycin, and kanamycin, have four to five groups, and neomycin, which is the most toxic, has six free amino groups. Changes in dosing to once-daily administration have evolved to avoid nephrotoxicity. Once-daily dosing
with monitoring of trough levels may enable avoidance of significant renal toxicity, even in elderly patients (
64). However, with prolonged treatment, differences between once-daily and twice-daily dosing diminish (
65). The toxicity of aminoglycosides may be potentiated by ischemia or other drugs, including thalidomide (
66).
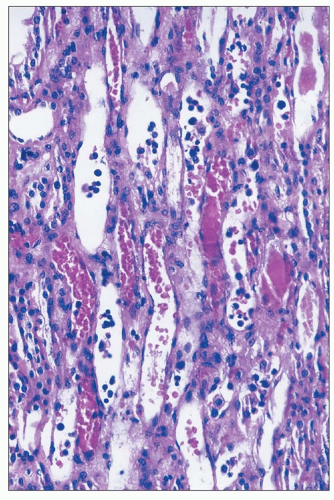
Gentamicin is a broad-spectrum antibiotic that has intermediate nephrotoxicity, and kanamycin also has an intermediate potential for nephrotoxicity. In humans, gentamicin alone may cause elevation of serum urea nitrogen (SUN) and sCr, although the incidence is difficult to assess because of a variety of concomitant clinical variables, including advanced age, presence of preexisting renal damage, or administration of other drugs that are potentially nephrotoxic. The frequency with which renal toxicity is reported varies from study to study, in part because of variable criteria for defining significant elevations in SUN and creatinine. Incidences ranging from 8% to 37% have been reported. Identified risk factors include advanced age, poor nutritional status, severe systemic illness, and administration of other drugs, including amphotericin B, vancomycin, methicillin, or cephalosporins, which are themselves potentially nephrotoxic.
Onset of a detectable rise in sCr is typically delayed for 8 to 10 days from initiation of therapy. Renal failure is usually mild, and most patients recover. Enzymuria may be detected in cases without elevations in sCr, suggesting the presence of subclinical injury in many patients. Occasional cases have been reported in which proximal tubular dysfunction is severe enough to produce Fanconi syndrome (
67). Neomycin is the most nephrotoxic of the aminoglycoside antibiotics. Because it is poorly absorbed from the gastrointestinal tract, it is used largely as a bowel-sterilizing drug. Neomycin also has been used parenterally and has caused deafness and renal damage. ARF, usually of an oliguric type, has been reported; recovery has been reported in most patients (
68,
69). ARF occurs most commonly after intravenous or intramuscular administration of the drug, although it has been recorded after oral administration as well (
69).
Amphotericin B Nephrotoxicity is the side effcect that most commonly limits the use of this important antifungal agent. Renal insufficiency is frequently observed, with a fall in the GFR and renal blood flow. In one large prospective series of patients being treated for cryptococcal meningitis, 26% had an increase in sCr level of more than 2 mg/dL (
70). Such renal failure is usually reversible, but renal function may be permanently impaired in 40% of patients who receive more than 5 g of amphotericin (
71). In addition, there is a defect in acid excretion by the tubules, resulting in renal tubular acidosis (
72), which may precede a significant fall in the GFR and is generally reversible. A common side effect is an impaired ability to concentrate urine (
73); this may be present without azotemia. Liposomal and lipid complex formulations may reduce nephrotoxicity (
74). The different formulations are probably comparable (
75).
Cephalosporins While acute (proximal) tubular injury is rare with the penicillins and uncommon with the current generation of cephalosporins, it is a greater risk with the penems. The cephalosporin group of antibiotics comprises several “generations” of these useful agents, defined on the basis of antimicrobial activity. The first generation includes cefazolin, cephalothin, and cephalexin. Cefamandole, cefonicid, cefuroxime, cefaclor, cefoxitin, and cefotetan are second generation, while the third generation includes ceftazidime, cefotaxime, and ceftriaxone. Cefepime is a fourth-generation cephalosporin that is more resistant to β-lactamase than the previous agents. Many of these drugs may be nephrotoxic (
76). Cephaloridine and cephaloglycin are the most toxic of the group and are no longer used clinically in the United States but are used experimentally for toxicity studies. On the other hand, ceftazidime and cefepime are not nephrotoxic.
The toxic cephalosporins are most likely to produce renal failure in patients with preexisting renal insufficiency, in those with drug overdose, and in those receiving other antibiotics or furosemide, probably related to the ability of furosemide to prolong the half-life of the cephalosporins. Many of the patients reported to have nephrotoxicity owing to cephalosporins are acutely ill with severe infections, and many are elderly. Cephalothin given alone or with gentamicin, tobramycin, or other agents can cause ARF in humans or can worsen preexisting renal insufficiency. The ARF is usually reversible. Cephalexin is less likely to cause nephrotoxicity, but acute renal dysfunction has been reported, with “acute tubular necrosis” (
77,
78). ARF with tubular proteinuria has been described with a combination of ceftriaxone and acyclovir (
79) and cefodizime and vancomycin (
80).
Polymyxin B and Colistin Polymyxin B and colistin (polymyxin E) are older antibiotics that are reemerging for treatment of multiple-drug-resistant gram-negative bacteria and are used for “salvage” therapy in critically ill patients. These antibiotics have well-recognized nephrotoxicity. At lower doses, proteinuria, casts, and hematuria may be seen, and at higher doses, renal failure occurs. Reduction in dosing, avoidance of coadministration of other nephrotoxic agents, and other supportive measures likely explain a lower incidence of nephrotoxicity in more recent clinical series compared to older reports (
81,
82). Incidence of nephrotoxicity in recent studies has been 10% to 24%, with comparable toxicity for colistin and polymyxin B regimens (
83,
84,
85,
86). When there is preexisting impaired renal function, smaller doses can produce renal symptoms. Renal failure may occur with oliguria. Recovery is usual after withdrawal of the drug.
Vancomycin Vancomycin is a glycopeptide antibiotic that has been associated with nephrotoxicity and ARF since its introduction (
87), limiting clinical use of the drug until the advent of methicillin-resistant
Staphylococcus aureus (MRSA) and other drug-resistant organisms. Nephrotoxicity was initially reported at low rates of ≤5% with standard dosing (
88), though higher rates were reported with use of concomitant nephrotoxic agents (
89). With newer recommendations for use of higher doses for MRSA and hospital-acquired infections, increased rates of nephrotoxicity have been reported over the past decade (
90,
91,
92). Risk factors include African American race, initial trough level, duration of treatment, and concomitant aminoglycoside use.
IMMUNOSUPPRESSIVE/IMMUNOMODULATORY AGENTS
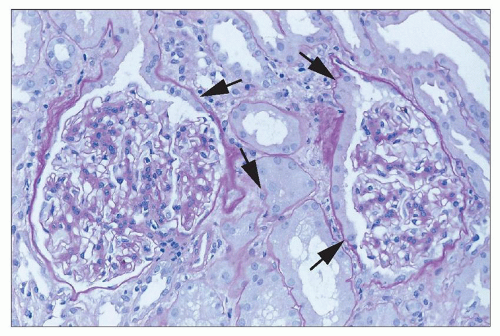
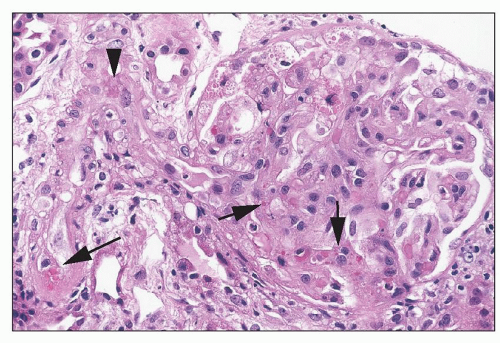
Cyclosporine Cyclosporine (CsA) is widely used in the prevention and treatment of transplant rejection and to treat autoimmune disease. The major side effect is nephrotoxicity, which is to some extent dose dependent. Both acute and chronic toxic effects have been described (
93). With nephrotoxicity broadly
defined to include an asymptomatic mild decline in the GFR, it is likely that many patients treated with immunosuppressive doses of CsA experience nephrotoxicity. When more overt CsA-induced renal failure is superimposed on mild functional toxicity, it may manifest in the form of one or more clinical syndromes: acute reversible renal functional impairment, delayed renal allograft function, tubular cell effects, acute vasculopathy (thrombotic microangiopathy), and chronic nephropathy with interstitial fibrosis.
The occurrence of
acute reversible renal failure, while not absolutely related to circulating drug levels, is generally seen with serum levels rising above 200 ng/mL and is common at drug levels above 400 ng/mL. Other features may include hyperuricemia, hyperkalemia, hypomagnesemia, sodium retention, and concentrating defects (
94,
95,
96). These relatively high levels are seen more commonly in heart and liver allograft patients than in patients with renal allografts. ARF may be severe, with polyuria or oliguria (and even rarely anuria). In some cases, renal functional impairment can be rapidly reversed when CsA dosing is reduced (
97). This rapid return of function is evidence that there is no direct tubular toxicity, as is the low fractional excretion of sodium, which indicates intact tubular reabsorption. In early phases, the underlying vasoconstriction can be reversed by dopamine (
98). Cyclosporine can also produce significant injury to proximal tubule epithelium, potentially related to direct effects as well as to ischemic injury due to prolonged vasoconstriction. In this setting, renal dysfunction is not rapidly reversible on reducing dosage of the drug.
Cyclosporine also has a propensity for producing endothelial cell damage, which can lead to thrombotic microangiopathy. Glomerular thrombi and thromboembolic complications have been described in several series, and a hemolytic uremic type of syndrome (HUS) has been reported, initially in bone marrow transplant recipients and subsequently in other contexts as well (
99,
100). There may be ischemic tubulointerstitial changes downstream from involved vessels.
Myers et al. (
101) were the first to show fibrosis with cyclosporine treatment, and many others have drawn attention to the fact that chronic nephropathy with striped interstitial fibrosis may occur following long-term CsA therapy, particularly in cardiac and other solid organ allograft recipients, as well as in patients receiving chronic CsA for autoimmune disease (
102). Proposed risk factors include episodes of clinical toxicity, high CsA trough levels, concurrent administration of other nephrotoxic drugs, acute rejection episodes and therapy, and high variability in CsA levels (
103,
104). Myers et al. showed significant reductions in the GFR in cyclosporine-treated patients to approximately 50% of that in azathioprine-treated patients (
101,
105). Patients may also have severe hypertension, mild proteinuria, and evidence of tubular dysfunction. A similar long-term reduction in the GFR has also been reported in liver allograft recipients (
106), and comparable changes have been reported in the kidneys of pancreas transplant recipients as well (
107). Even low-dose CsA therapy for psoriasis may effect long-term changes (
108,
109). This type of chronic cyclosporine toxicity may not be reversible. Risk factors for the development of chronic cyclosporine nephrotoxicity include previous episodes of ARF, high-dose treatment, and (for heart transplant patients) increasing age (
110,
111).
Tacrolimus Tacrolimus (FK506) produces a spectrum of nephrotoxicity very similar to that of CsA (
93,
94) and is generally dose dependent; toxic reactions are common at or above 20 ng/mL (
112) but can occur even when trough levels have been in a lower range (
103). Reversible renal dysfunction has been reported with the use of FK506 for prevention of graft versus host disease in bone marrow transplantation and in renal and nonrenal solid organ allografts. Tacrolimus may have a lower nephrotoxic potential than cyclosporine in renal allografts, with less reduction in blood flow (
113), and lower sCr and/or higher GFR at doses with comparable efficacy (
114,
115,
116). Better graft survival has been reported in renal allografts (
116,
117), and less CRF may occur in other solid organ allografts with tacrolimus versus cyclosporine (
118,
119,
120). In addition to induction of posttransplant diabetes, patients may develop hypertension (
121). Higher incidences of urinary tract infection, of pyelonephritis, and of polyoma virus infection (
122) have been reported as well, perhaps owing to the more potent immunosuppressive activity of the drug.
Intravenous Immunoglobulin Intravenous immunoglobulin (IVIG) may produce ARF (
123,
124). Addition of sugar excipients, and especially sucrose, to IVIG formulations has reduced side effects of pain, fever, chills, and fatigue but may increase the frequency of ARF. Renal failure may be attenuated by slowing the rate of infusion. Renal function generally returns to normal with discontinuation of the drug. Switching to a D-sorbitol-stabilized formulation may prevent toxicity (
124). Avoidance of sucrose-stabilized formulations is recommended in patients receiving other nephrotoxins, in the elderly, in those with preexisting dysfunction, and in diabetics.
Sirolimus Nephrotoxic effects of sirolimus have been reported in transplant and native kidneys. Delayed graft function is more common in series of patients treated with sirolimus peritransplant (
125,
126); another study demonstrated that sirolimus-treated patients were half as likely to resolve delayed graft function (
127). A few cases of acute oliguric renal allograft failure associated with combined use of FK506 and sirolimus have been described, apparently owing to ATI (
128). In one study of high-risk renal allograft recipients, FK506-treated patients on reduced sirolimus (5 to 10 ng/mL) had a significantly higher incidence of biopsy-proven FK506 toxicity (
129). Severe acute renal dysfunction with tubular injury with myoglobin casts has been reported in renal allograft recipients, with ATI, with myoglobin casts noted only in the cohort treated with rapamycin (
130), some with elevated creatine phosphokinase and/or serum myoglobin levels. ARF/AKI has also been reported in series of patients treated with rapamycin for chronic glomerulopathy (
131); renal biopsies were not performed, but most recovered function after discontinuation of the drug. Some cases of acute renal dysfunction caused by sirolimus have been associated with thrombotic microangiopathy (
132).
ANTINEOPLASTIC AGENTS
Several antineoplastic agents produce toxic effects in the kidney. Immunotherapeutic agents, discussed earlier, are among them. In addition, antineoplastic agents that lead to rapid tumor lysis may cause hyperuricemia, with precipitation of uric acid in renal tubules; this syndrome may be largely avoided by hydration and careful monitoring of the patient. Specific agents that are toxic to the kidney are discussed here.
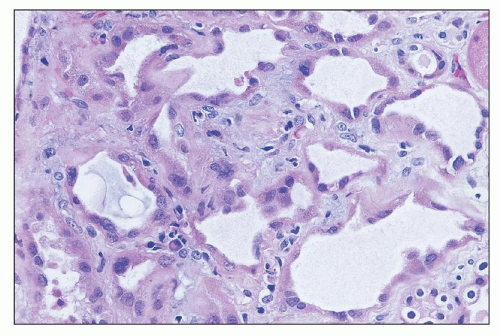
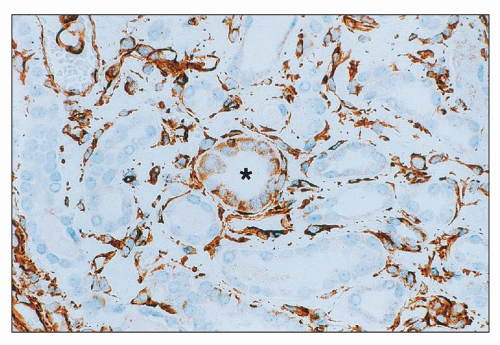
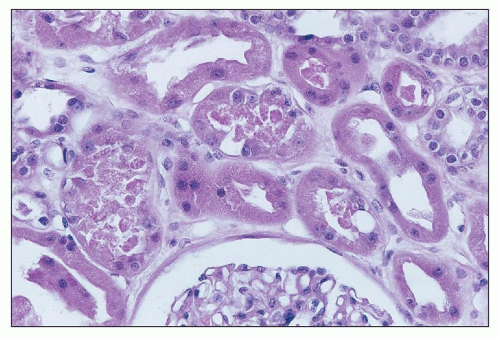
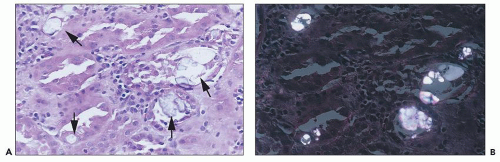
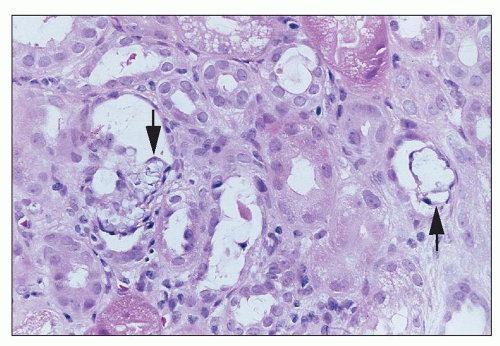
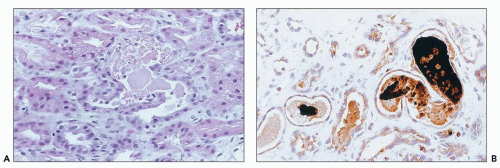
Cis-Platinum Cis-platinum is a chemotherapeutic agent that frequently produces nephrotoxicity and is widely used in animal models of toxic tubular injury. Cis-platinum nephrotoxicity is dose related. In early studies, it was reported in 25% to 30% of patients on single-course therapy and 50% to 75% of patients on multiple courses (
133,
134) and remains high, affecting about one third of treated patients (
135). Patients show gradual signs of elevations in SUN and sCr. Polyuria is a prominent early clinical feature, but even oliguric ARF may be seen. Other presenting symptoms include proteinuria, hyperuricemia, enzymuria, glycosuria, and electrolyte disturbances reflecting tubular dysfunction (
136,
137). Aggressive hydration, administration of diuretics, or coadministration of thiosulfate or thiophosphate reduces renal toxicity, and novel cytoprotective strategies based on understanding of pathophysiology are being tested in animal models (
136,
137,
138). Delay of dosing is recommended if renal toxicity occurs. Recovery of renal function following cessation of therapy is the rule, but it may be delayed and incomplete, and subclinical dysfunction may persist (
139). Chronic renal dysfunction is best predicted by the cumulative dose administered. Newer platinum derivatives, including carboplatin, spiroplatin, iproplatin, and oxaplatin, and liposome-entrapped platinum compounds appear to have limited nephrotoxicity. However, there is still a degree of nephrotoxicity with some of these formulations (
138). Nephrotoxicity may be exacerbated by combination therapy with other agents such as Taxol (
140).
Other Chemotherapeutic Agents Nitrosoureas also produce nephrotoxicity. Streptozotocin, a nitrosourea compound, is toxic to pancreatic beta cells and is used to treat metastatic islet cell carcinoma, carcinoid tumors, and lymphoma. Up to 75% of patients experience some degree of nephrotoxicity with prolonged administration (
141,
142). The alkylating agent cyclophosphamide has only transient effects on water excretion, increasing urine osmolarity and decreasing plasma osmolarity. However, its analog, ifosfamide, has significant renal toxicity. Renal proximal tubular dysfunction is the most common effect, and features of Fanconi syndrome and related electrolyte abnormalities, which may be severe, have been reported (
137,
138). Distal renal tubular acidosis occurs rarely. Mild decreases in the GFR are common, but severe ARF may occur as well, and irreversible chronic renal failure or continued deterioration after therapy has also been described (
143,
144). The major risk factor for toxicity is total dose of the drug (
143). Other risk factors include age less than 5 years, previous exposure to cisplatin, underlying renal impairment, or tumor infiltrates in the kidney (
145). Use of thiophosphates may reduce toxicity (
146).
Other chemotherapeutic agents may also be nephrotoxic (
137,
138). High-dose therapy with mithramycin or methotrexate can result in renal failure, the latter via precipitation of methotrexate and 7-hydroxymethotrexate crystals in tubules. Azacitidine can produce renal symptoms and Fanconi syndrome or mild subclinical tubular dysfunction, which may necessitate bicarbonate and electrolyte supplementation. Imatinib and diaziquone can also produce Fanconi syndrome and AKI. Zolendronate, a bisphosphonate used in conjunction with chemotherapeutic agents, has also been associated with ARF and should be avoided in patients with severe underlying renal disease.
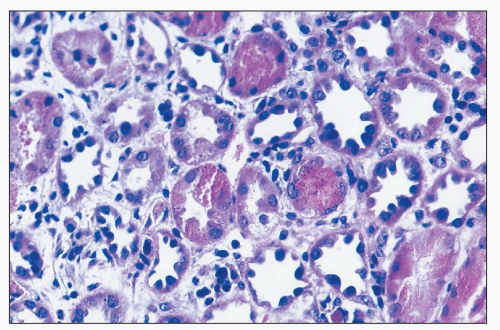
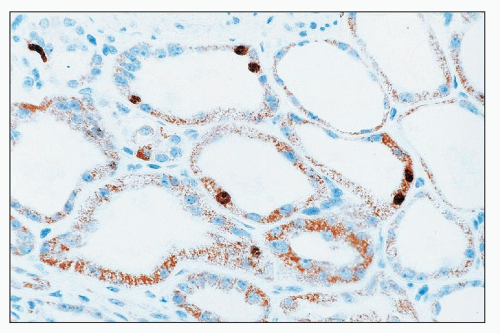
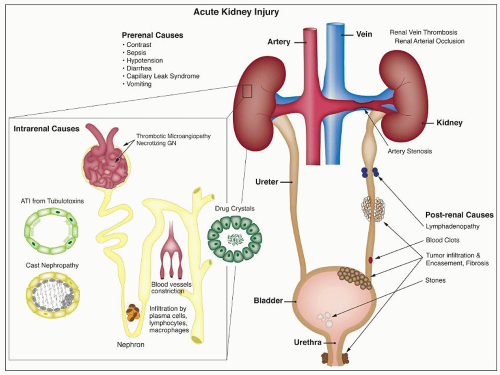
RADIOLOGIC CONTRAST MEDIA
Renal failure is an important complication of contrast media administration; the reported incidence of radiocontrast nephrotoxicity (RN) varies between 2% and 70%, averaging 5% to 10%. In the United States and Europe, RN has been reported to be the cause of 10% of hospital-acquired ARF (
147). Differences in reported incidence are in part the result of issues with the definition of RN, optimally defined as “acute impairment in renal function following exposure to radiographic contrast materials.” This impairment is measured by a rise in sCr by most investigators. However, the degree of change in sCr that is considered to be diagnostic of RN shows great variation. Some prospective studies, which measured the sCr levels at regular intervals, diagnosed RN even after relatively small increases. Thus, some of these studies may overestimate the incidence of clinically significant RN. Urinary levels of tubular cell enzymes and markers of oxidative stress rise in the urine of patients with RN (
148,
149).
Certain underlying conditions predispose to the development of RN; the most important of them is preexisting renal insufficiency (
150,
151). Moore et al. (
152) demonstrated that the incidence of RN in patients with baseline sCr levels between 1.5 and 1.9 mg/dL was 4.7%, the incidence for those with sCr levels between 2.0 and 2.4 mg/dL was 14.3%, and for levels between 2.5 and 2.9 mg/dL, it was 20%. Analogous findings were reported in a large study, with incidence of RN (rise in sCr of more than 0.5 mg/dL) ranging from 2.4% with sCr of 0.1 to 1 mg/dL to 30.6% for sCr above 3 mg/dL (
150). A recent study in general ICU patients (
153) using only iodinated nonionic low-osmolar or iso-osmolar contrast found an incidence of AKI of 16.4% using standard criteria (22.2% using KDIGO criteria); AKI was stage 3 (severe) in 25% of those who developed AKI. In contrast, with low-risk nonemergent CT, RN is uncommon among outpatients with mild baseline kidney disease (
154). Dehydration is a risk factor for RN, which is not surprising because dehydration, and thus hypovolemia, may potentiate the development of renal failure owing to any insult. Effective prophylactic measures, such as rehydration, alleviate this problem. The efficacy of prophylactic hemodialysis and hemofiltration to reduce the incidence of RN in high-risk groups is controversial (
151). A variety of protective measures have been proposed, including hydration and sodium bicarbonate,
N-acetyl cysteine, combination therapy, and statin therapy (
155).
Diabetes and
multiple myeloma are also risk factors for RN. However, it appears that neither condition represents a higher risk if renal function is normal. Identified risk factors for contrast-induced nephropathy after coronary intervention studies include CHF, hypotension, intra-aortic balloon pump, age greater than 75 or 80 years, anemia, diabetes mellitus, contrast volume, and high preprocedural sCr (
156). Whether
dosage and
route of administration are independent risk factors is a matter of debate. Some studies found a significant correlation between the volume of administered contrast media and the degree of nephrotoxicity, particularly in patients with underlying renal disease such as diabetes mellitus and in the setting of reduced renal function. Other studies did not confirm this relationship. These differences probably reflect biases in selection of patients. Dose may not be a significant risk factor in patients with normal renal function, but dosage as an independent risk factor has not been investigated in most published studies. An equation, to determine maximum acceptable
contrast dose, 5-mL contrast volume × body wgt (kg)/baseline sCr (mg/dL), has been developed (
157). Several recent studies confirm that exceeding this threshold increases the risk for contrast-induced nephropathy (
158,
159), at least in percutaneous coronary intervention studies. Patients who develop RN reportedly have more frequent adverse events, including myocardial infarction, prolonged hospital stay, worse kidney function at discharge, and higher mortality (
153,
160).
Following the introduction of nonionic (low-osmolality) contrast media, some studies suggested that such media are less nephrotoxic than the conventional ionic (high-osmolality) contrast media. However, several prospective clinical studies comparing the nephrotoxic effect of low- and high-osmolality contrast media did not find differences in the incidence of nephrotoxicity (
152). The randomized prospective multicenter Iohexol Cooperative Study group (
161) found that in patients with normal renal function, the incidence rates of RN were not different between patients undergoing cardiac angiography using ionic (sodium diatrizoate) and those patients receiving nonionic (iohexol) contrast media. In contrast, patients with preexisting renal impairment receiving ionic contrast material were 3.3 times more likely to develop RN than patients receiving nonionic contrast material. Thus, it appears that the considerably cheaper conventional ionic contrast media can be safely used in patients with normal renal function, but ionic contrast media should be used with caution in those with preexisting renal insufficiency. Iso-osmolar (e.g., iodixanol) and (less expensive) low-osmolar (iomeprol, iopamidol, ioversol) contrast agents have also been compared in randomized trials. Most show some trend to lower rates of contrast-induced AKI with the iso-osmolar agent, but generally, the difference was not significant (
162,
163,
164). However, in those receiving high contrast volume (
163) or in diabetic patients (
164), there may be a lower risk with the iso-osmotic agent.