hemorrhage. Unless Goodpasture syndrome is restricted to one pathogenic category, the term does not specify a clinically and pathogenetically distinct entity.
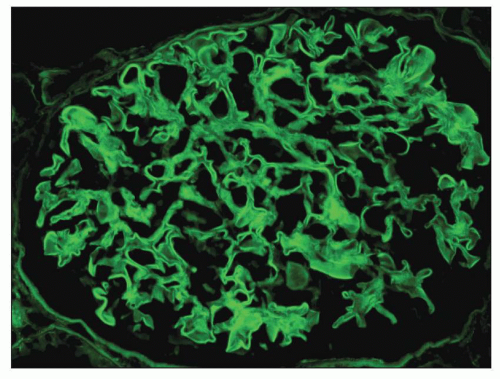 FIGURE 15.1 Global linear staining for IgG indicative of diffuse binding of anti-GBM antibodies to type 4 collagen in glomerular basement membranes. (FITC anti-IgG.) |
of immune complex disease caused by in situ binding of anti-GBM antibodies to GBM antigens, but it is traditionally separated from other forms of immune complex glomerulonephritis when categorizing CGN. Throughout this chapter, the term immune complex glomerulonephritis is used for glomerulonephritis caused by immune complexes other than anti-GBM immune complexes.
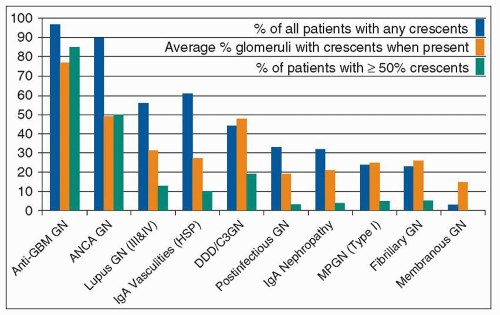
crescent formation than immune complex glomerulonephritis (Table 15.2 and Fig. 15.4) (18,19). This finding suggests that the pathogenic mechanisms that underlie anti-GBM and ANCA glomerulonephritis are more destructive than the mechanisms that underlie immune complex glomerulonephritis. However, some categories of immune complex glomerulonephritis tend to have a greater frequency of crescent formation than do others. In general, the extent of glomerular subendothelial, as opposed to subepithelial or mesangial, immune complex localization correlates with severe inflammation and crescent formation among patients with immune complex glomerulonephritis. This suggests that the proximity of subendothelial immune complexes to the inflammatory mediator systems in the circulation is more effective at causing the glomerular injury that results in crescent formation than are immune complexes in the mesangium or subepithelial zones of glomeruli. This could in part explain the severity of anti-GBM glomerulonephritis because the autoantibodies form immune complexes in situ with the GBM antigens at the subendothelial interface between the GBM and the plasma.
TABLE 15.1 Frequency of different types of Crescentic Glomerulonephritisa in renal biopsy specimens evaluated by the University of North Carolina Nephropathology Laboratory | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
TABLE 15.2 Frequency of glomerular crescents, necrosis, and endocapillary hypercellularity in different types of glomerular disease evaluated by the University of North Carolina Nephropathology Laboratory | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 15.3 Features at the time of presentation of different types of Crescentic Glomerulonephritisa Evaluated by the University of North Carolina Nephropathology Laboratory | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
smokers compared to 17% of the general population, and only 14% had never smoked compared to 56% of the general population (P < 0.001) (38). Savage et al. (33) observed that there is a tendency for young men with anti-GBM disease to present with Goodpasture syndrome (i.e., combined pulmonary hemorrhage and nephritis) and for older women to present with glomerulonephritis alone. We have observed a similar pattern in the patients at the University of North Carolina. Overall, however, the male-to-female ratio is equal (see Table 15.3). Anti-GBM glomerulonephritis is rare in African Americans.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree