quite liberally and include cases of glomerulonephritis with a preceding infection, but the infection is still present. Such cases include glomerulonephritides associated with staphylococcal infection (see section on Glomerulonephritis associated with staphylococcal infections). However, if we consider a glomerulonephritis following an infection postinfectious, then even HIV-associated nephropathy or hepatitis C-related cryoglobulinemia could be considered postinfectious glomerulonephritis, which would clearly be inaccurate. In our opinion, poststreptococcal glomerulonephritis is the best example for postinfectious glomerulonephritis. Most other infection-related glomerulonephritides are not postinfectious; they are associated with ongoing infection.
this variability. Of all children infected with the various strains of streptococci, it appears that less than 2% show clinically obvious signs of acute glomerulonephritis.
resistance in GAS. Certain GAS organisms have surface receptors that bind selectively to the key fibrinolytic enzyme, plasmin (73). The bacterium-bound plasmin retains its enzymatic ability to cleave substrates and hydrolyze a fibrin clot, which may in part contribute to its tissue-invasive properties (73). GAS infection can be diagnosed and monitored with many laboratory procedures that detect the organism, its antigens, or its antibodies.
TABLE 10.1 Incidence and prevalence of PSAGN worldwide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
contained in the most conserved region of the M protein have yet been possible (81,82). Molecular typing of the M protein has been used to investigate the molecular epidemiology of GAS as well as group C and G streptococcal disease. A systematic review of the global distribution of GAS M types revealed that the epidemiology of GAS disease in Africa and the Pacific region seems to be different from that in other regions, particularly the United States and Europe. In Africa and the Pacific regions, there is more diversity of M types. The M types (including 1, 4, 6, and 12) that are more common in the highincome countries were found to be less common in Africa and the Pacific region. This has implications for the development of multivalent GAS vaccines. One vaccine may not provide good coverage worldwide (81). Nontypeable GAS organisms also have been cultured from patients with acute glomerulonephritis, which presumably represent unclassified nephritogenic strains (20). It is likely that multiple factors of the bacteria and the host contribute to the differences in the attack rates for different strains of streptococcal organisms. Numerous proteins/antigens were described that are characteristic of nephritogenic strains of streptococci. These nephritogenic proteins are discussed later in this chapter.
is required. A preceding infectious episode (such as pharyngitis, tonsillitis, mastoiditis, peritonsillar abscess, otitis media, or pyoderma) is the sine qua non for clinical diagnosis of PSAGN (93,94). Skin infection also may lead to nephritis (13,16,17,22,31). This is most often associated with epidemics, particularly in humid warm climates. The offending organism is virtually always a GAS; types 12, 4, 1, and 49 appear to be the most typical nephritogenic types.
of Jennings and Earle (115). Proteinuria may persist for longer periods, but complete clinical recovery has been noted after proteinuria has been maintained for as long as 26 months (115). McCluskey and Baldwin (114) described a well-documented case of one patient with disappearance of proteinuria after 6 years. Symptoms, including proteinuria, hypertension, and renal insufficiency, are more severe in adults and, in particular, in the elderly with postinfectious glomerulonephritis (2).
may have tiny red speckles caused by red blood cells in the lumens of the Bowman space and tubules.
TABLE 10.2 Atypical features that suggest a need for renal biopsy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
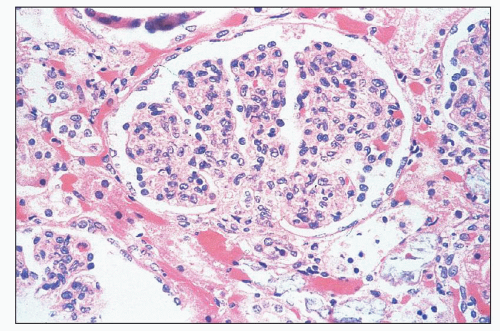
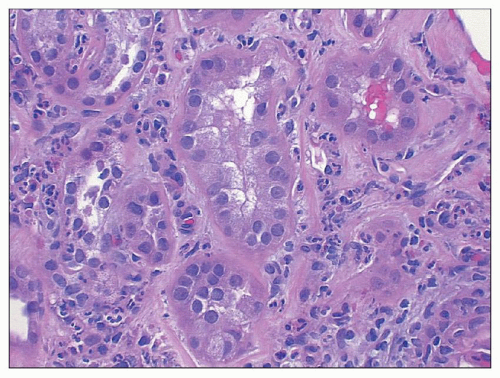
sometimes be mild thickening visible on light microscopy. The combination of expansion of the lobules, hypercellularity of the tuft, and localized thickening of the glomerular capillary walls may produce a picture mimicking MPGN. Ultrastructural and immunofluorescence studies and clinical findings at the time of biopsy and follow-up allow easy separation of these morphologically and clinically distinct entities.
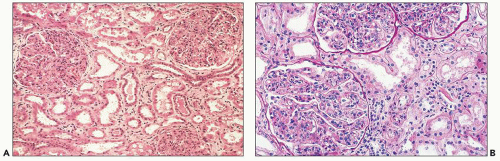
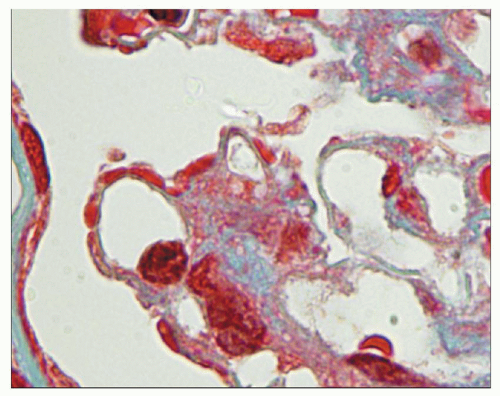
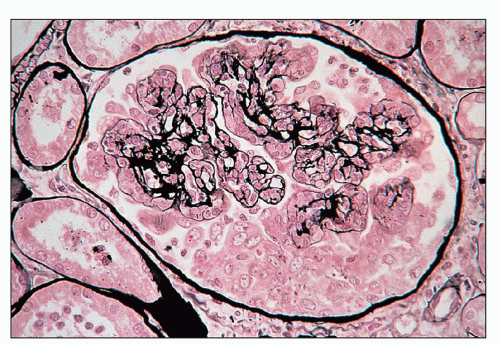
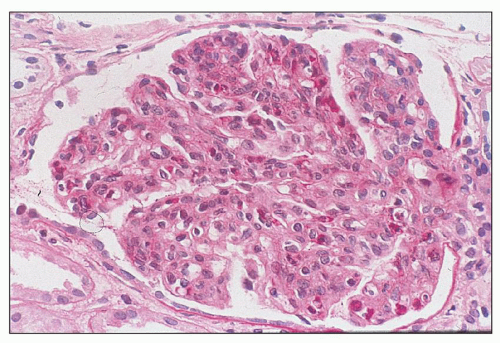 FIGURE 10.4 This glomerulus shows a broadening of the lobules, increase in cellularity with moderate numbers of neutrophils with segmented nuclei, and reduction of the capillary lumens. (PAS, ×400.) |
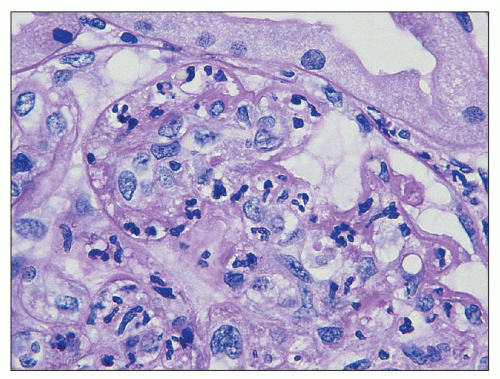 FIGURE 10.5 Acute diffuse proliferative glomerulonephritis with considerable infiltration of the glomerulus by neutrophils, which is common in acute postinfectious glomerulonephritis. (PAS, ×1000.) |
hypercellularity. The old term proliferative glomerulonephritis implies that the increased cellularity is restricted to native glomerular cells, either endocapillary cells (endothelial or mesangial) or extracapillary cells (visceral and parietal epithelial cells). However, current evidence suggests that much of the glomerular hypercellularity (as well as some of the crescent formation in the Bowman space) stems from infiltrating leukocytes from the peripheral circulation. Langhans (8) initially proposed that the major cause of the cellular increase in the glomerular tuft was a proliferation of endothelial cells. This concept was responsible for the designation endocapillary proliferative glomerulonephritis.
mesangial hypercellularity, but less exudative change, had fewer esterase-positive cells. It was suggested that early glomerular hypercellularity is owing to an influx of blood-borne cells, but at a later stage, it is caused mainly by proliferation of intrinsic glomerular cells (62,64). Magil et al. (62,64) verified the presence of glomerular intraluminal monocytes that correlated positively with the presence of deposits. They also described dissection of the glomerular endothelium from the capillary wall adjacent to deposits by the monocytes. Ferrario et al. (58) showed that the degree of proteinuria correlated well with the degree of glomerular mononuclear cell infiltration.
Second, many cases termed chronic latent glomerulonephritis may not be resolving/resolved PSAGN, in our opinion, but rather represent a nonspecific histologic pattern associated with various renal injuries unrelated to previous infections (132). In one long-term follow-up study (more than 5 years) of 26 patients suffering from PSAGN, Buzio et al. (133) found diffuse mesangial hypercellularity in those patients with persisting urinary albumin or proteinuria.
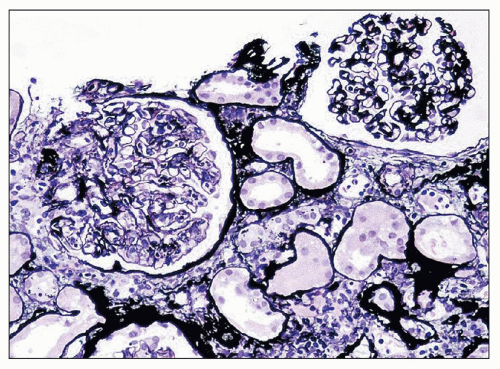
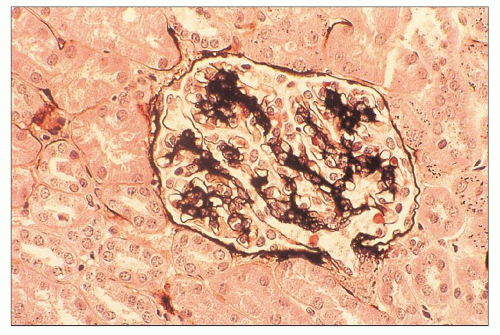 FIGURE 10.10 Resolving PSAGN shows mesangial expansion. Increased mesangial cellularity and mesangial matrix increase may persist for years. (Jones methenamine silver, ×200.) |
and scattered regions of interstitial fibrosis. Usually, however, the interstitial changes are not remarkable or severe. As noted earlier, interstitial changes may be found in relation to tubular changes (137). Bohle et al. (138), using morphometric methods on tissue sections, showed that the level of serum creatinine correlated with the increase in interstitial volume. They explained this finding on the basis of a reduction of renal blood flow and, hence, the GFR, brought about by compression of the postglomerular vasculature.
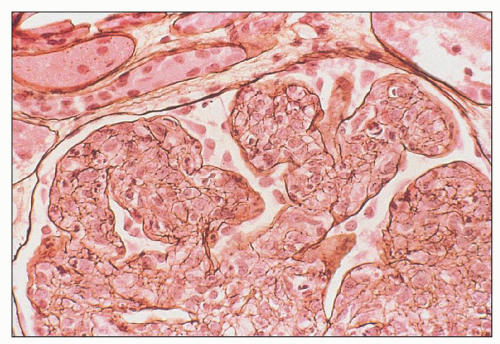
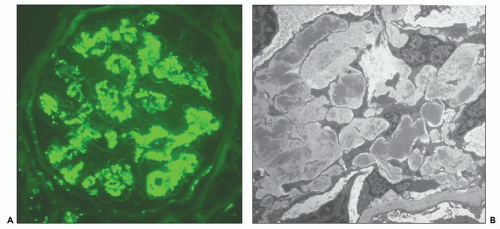
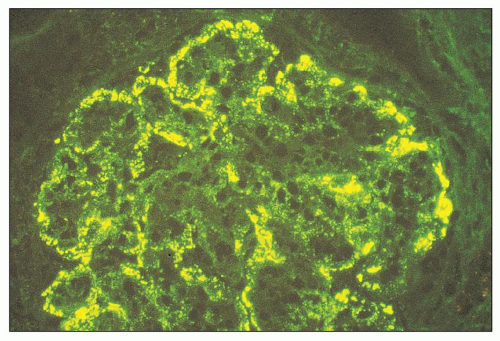 FIGURE 10.12 Immunofluorescence microscopy for IgG in a patient with PSAGN shows a coarsely granular pattern along the capillary walls and a less prominent granular mesangial pattern. (×400.) |
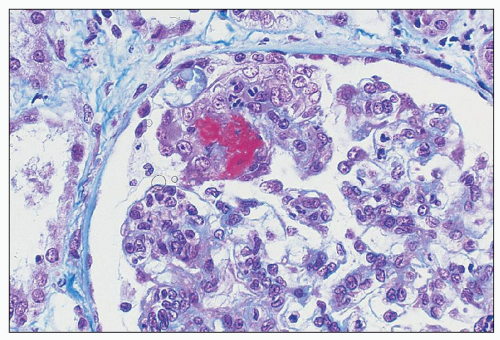
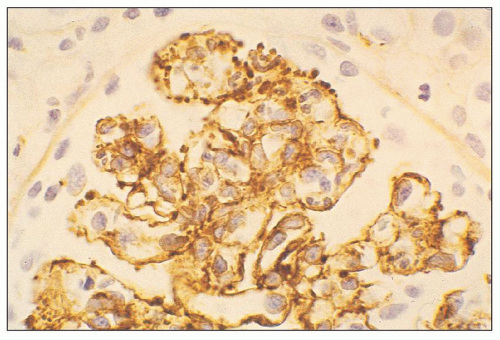
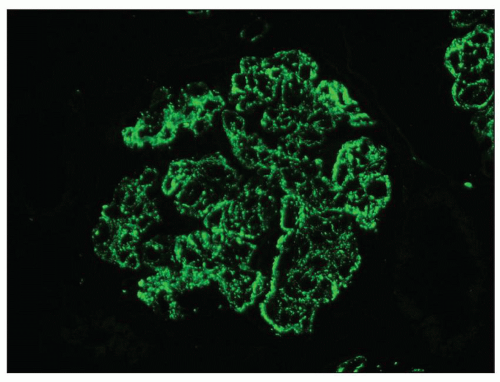 FIGURE 10.13 “Lumpy bumpy” coarsely granular C3 deposits along the glomerular capillary loops in PSAGN. (×400). |
Fig. 10.17). The deposits are generally noted in the mesangial matrix of the glomerulus and are accompanied by mesangial hypercellularity.
The starry sky pattern was noted in four of five patients with a crescentic pattern and six of seven patients with a chronic course (154). There is no evidence so far that different etiologic factors are responsible for these three subtypes (148). The individual immune response of the host and the stage of the disease are likely to play a role in their genesis. A diffuse granular pattern for IgG and, usually, C3 is also found in patients with subclinical glomerulonephritis (146,150,151) and in those with minimal urinary changes.
onset of disease (162). Sometimes, they persist for longer periods of time (163), but the clinical course in such cases is not clear. The fate of the glomerular subepithelial deposits has been studied by Tornroth (164), who has shown that the electron density (osmiophilia) of the deposits diminishes with time, so that electron-lucent regions are formed in the subepithelial zone that eventually disappear. The deposits may disappear by dissolution and passage into the blood or urinary ultrafiltrate or by pinocytotic removal by podocytes. Glomerular intramembranous electron-lucent regions have been seen in later biopsies (after 1 month) and, in some cases, these regions protruded toward the epithelium and were covered on that side by a thick layer of basement membrane-like material.
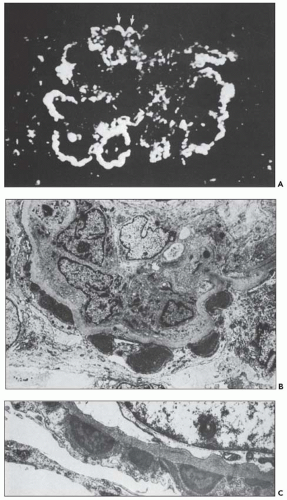
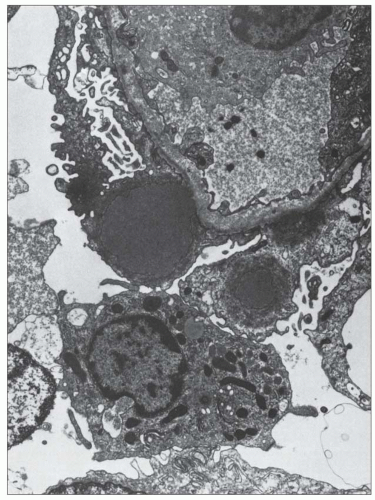
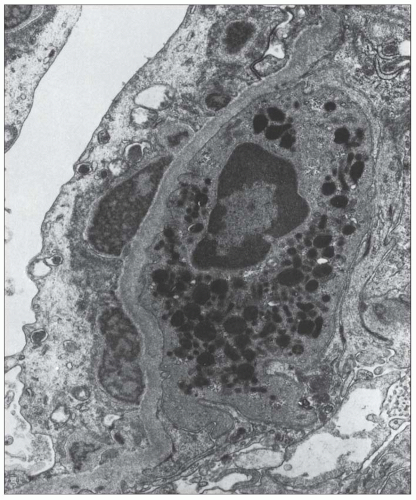
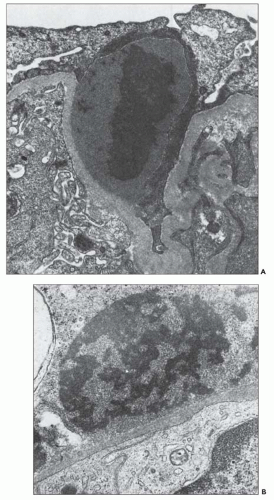
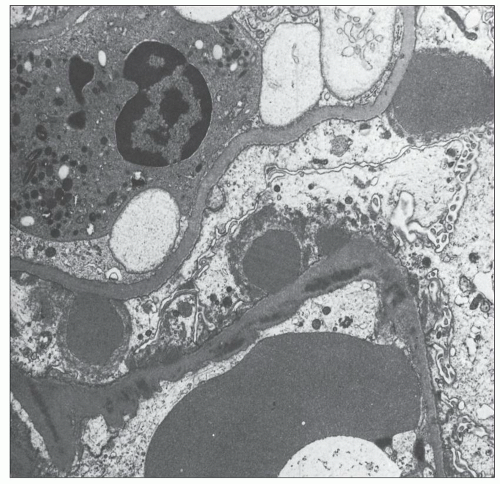
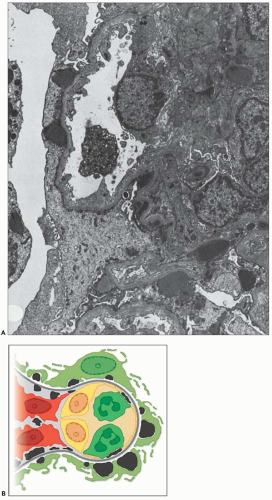 FIGURE 10.19 Electron micrograph and drawing showing the ultrastructural features of PSAGN. A: This electron micrograph shows a number of discrete electron-dense osmiophilic deposits in the subepithelial portions of the glomerular capillary walls. Some of the glomerular capillaries are narrowed or compressed, but one is patent. (Uranyl acetate and lead citrate, ×6930.) (Courtesy of Drs. William Murphy and Lillian Gaber.) B: Drawing depicting a single glomerular capillary with features of PSAGN including variably sized subepithelial, small subendothelial, and multiple mesangial electron-dense deposits (black), mesangial hypercellularity (red), endothelial hypercellularity (yellow), capillary margination of neutrophils (dark green), and effacement of podocyte foot processes (light green). Compare this drawing with features in the electron micrographs in Figures 10.15, 10.16, 10.17, 10.18, 10.19, 10.20, 10.21, 10.22, 10.23. |
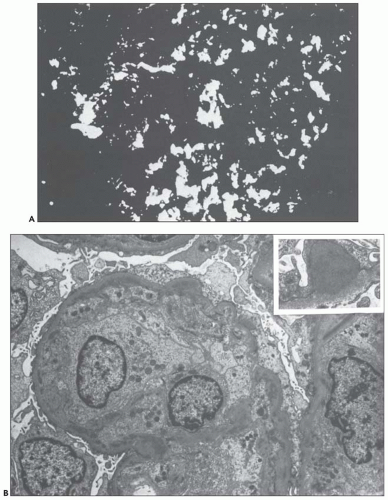
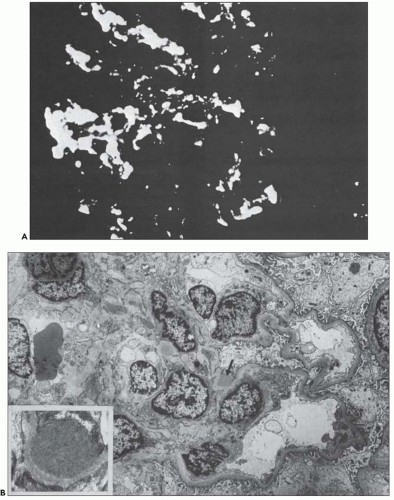
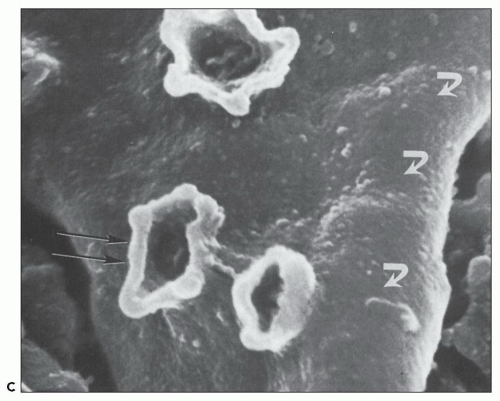
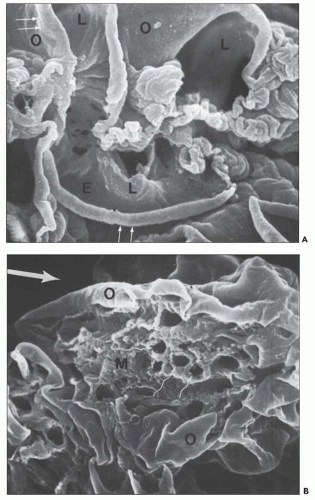 FIGURE 10.24 Scanning electron micrographs using the technique of Bonsib (170), in which the cellular elements have been removed by enzymatic digestion, leaving behind only the basement membrane, mesangial matrix, and other basement membrane-like substances. A: A segment of a normal glomerulus for purposes of orientation shows the open capillary lumen (L) and endothelial (E) aspect of the GBM (thin arrows). The subepithelial outer (O) surface of the GBM is also identified. B: A segment of a glomerulus from a patient with acute proliferation, as manifested by alterations in the mesangial matrix (M); holes (wavy white arrows) represent the former site of either proliferating cells or deposits. Orientation is provided by the outer (O) aspect of the GBM and urinary space (thick white arrow). C: A segment of a glomerulus from a patient with later changes seen in PSAGN, showing the subepithelial aspect of the GBM (curved white arrows) with reaction (dark arrows) to the site of deposit. (A, ×6375; B, ×2550; C, ×13,125.) (Courtesy of Dr. Steven M. Bonsib.) |
that are empty, reflecting the solubilization of the subepithelial immune complexes. The craters are uniform in size and shape. Every glomerulus studied contained at least several craters that were located at various sites within the glomerular tuft. The case from which Figure 10.24C was prepared was unique because of the presence of subepithelial humps several years after the acute nephritic episode and because of the large size of GBM craters.
Although some of these experimental manipulations produced histologic lesions somewhat similar to the disease pattern in man, most called for the periodic administration of the putative factors thought to be involved; this, of course, does not precisely mimic the gradual release of streptococcal products that probably occurs at the site of infection in the clinical condition in humans (85). Also, many of the experimental studies were performed at a time when electron and immunofluorescence microscopy and other biochemical determinations were not available, making it difficult to carry out an adequate comparison (85).
TABLE 10.3 Potentially nephritogenic streptococcal antigens | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
elevations of endostreptosin titers are generally diagnostic of PSAGN. Although low titers of antibody have been found in as many as 70% of normal individuals, significantly higher titers of antibodies are found in patients with poststreptococcal glomerulonephritis (200). Most patients with acute rheumatic fever do not have these high levels of antibody titer. Thus, Lange et al. (198) believe that elevated levels of antibody to endostreptosin are diagnostic of postinfectious glomerulonephritis and correlate well with the course of the pathologic disease process. Experimental studies by Cronin and Lange (197), using Wistar Furth rats, showed deposition of endostreptosin along the GBMs 1 day after injection of immunoaffinityisolated endostreptosin. Rats killed on days 8 to 12 showed increasing deposition of IgG and C3 with diminished staining for endostreptosin. No antiendostreptosin antibodies were detected in the sera in the first 3 days, whereas rats from day 4 onward had low levels of these antibodies. According to these authors, endostreptosin does not appear to be immunologically related to streptococcal exoenzymes or the streptococcal cell wall (197). Endostreptosin is similar to the preabsorbing antigen described by Yoshizawa et al. (188,201,218) and Holm et al. (228).
streptokinase gene (SKA1). Strains with deleted SKA1 gene did not cause glomerulonephritis in their mouse model (203,204). Studies conducted by Mezzano et al. (236) and others (237,238) have failed to make a strong connection between streptokinase and PSAGN. Mezzano et al. (236) did not find any unique reactivity to group A streptokinase in the sera of patients with PSAGN, and they also failed to establish the presence of streptokinase in renal biopsies early in the course of disease in 10 patients with PSAGN. Okada et al. (238) studied the major variable region of streptokinase genes of S. pyogenes strains isolated from patients with and without PSAGN. The major variable region of the streptokinase gene did not show any apparent relationship to poststreptococcal glomerulonephritis, suggesting that unique classes of streptococcal streptokinase do not play a role in the pathogenesis of PSAGN (238).
to be PSAGN, the absence of a low complement level indicates that the patient does not have PSAGN. It is worth noting that patients with postinfectious glomerulonephritis, not related to streptococcal infection, may have normal serum complement levels more commonly than patients with PSAGN (2). Serum complement levels rise to normal levels after several weeks and almost always return to normal within 6 weeks. In more than half the patients of Fischel and Gajdusek (251), normal serum complement levels had been attained within 3 weeks of the acute clinical onset of disease. All 32 patients studied by Cameron et al. (250) had regained normal serum complement levels within 4 months and all but 2 within 2 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree