Jan H.M. Tordoir
Vascular Access for Dialytic Therapies
Functional vascular access is needed for all extracorporeal dialytic therapies and remains the lifeline for patients with end-stage renal disease who need chronic intermittent hemodialysis (HD) therapy. The ideal HD access should have a long length of a suitable superficial vein for cannulation in two places more than 5 cm apart with a sufficient blood flow for effective dialysis, usually in excess of 400 to 1500 ml/min. A vascular access should have good primary patency, have a low risk of complications and side effects, and leave opportunities for further procedures in the event of failure. Ideally, a first access should be an arteriovenous (AV) fistula placed peripherally at the wrist. However, upper arm and lower limb access sites are increasingly used because the aging dialysis population, with multiple comorbidities, has poor and diseased arm vessels that may be unsuitable for the creation of a simple wrist fistula.
Vascular access should be performed with minimal delay by a surgeon experienced in vascular access creation and, wherever possible, in advance so that dialysis may start with permanent access rather than with use of a central venous catheter. Central venous catheter use should be minimized because of the increased risk of sepsis, the increased mortality, and the development of central venous stenosis or thrombosis, which compromises further access in the upper limbs. Unfortunately, many patients require a central venous catheter either to start dialysis or as a bridge between the failure of a permanent access and the creation of a new AV fistula.1
The need for revisional procedures because of access-related complications, including thrombosis, central venous obstruction, and ischemia, is increasing. A multidisciplinary approach to access creation and maintenance, involving nephrologists, interventional radiologists, access surgeons, and dialysis nurses, is mandatory to meet the burden of HD vascular access on health care facilities and costs.
Evaluation of the Patient for Vascular Access
The earlier a patient with chronic kidney disease (CKD) is seen by a vascular access surgeon, the better the chance for the patient to have a well-functioning access at the initiation of HD. An early decision on the type, side, and site of the first vascular access will be based on the following:
Preservation of veins during the earlier stages of CKD is crucial for the success of vascular access. Patients should be instructed to protect their veins, restricting blood sampling to the dorsum of the hand whenever possible.
Primary Autologous Vascular Access
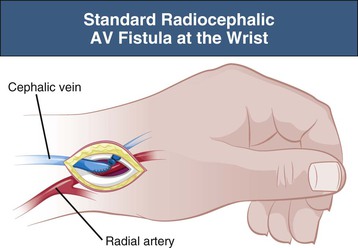
Radiocephalic Arteriovenous Fistula
A well-functioning distal radiocephalic AV fistula in the nondominant arm is the ideal permanent access for HD. This usually gives an adequate blood flow and a long length of superficial vein for needling. It also leaves proximal sites for further procedures in the event of failure. A distal radiocephalic AV fistula should be possible in a majority of incident patients but may be compromised if the cephalic and antecubital fossa veins are unusable because of thrombophlebitis from previous intravenous cannulas or venipunctures. For this reason, it is essential that these veins be avoided for intravenous cannulas, which should be restricted to the dorsum of the hand in all patients with CKD, except in the emergency situation when rapid access to the circulation is required.
A radiocephalic AV fistula is usually created at the wrist but can be created more proximally in the forearm if distal vessels are inadequate (Fig. 91-1). On occasion, three or four radiocephalic AV fistulas can be created at progressively more proximal sites in the forearm before a brachiocephalic AV fistula is created. The radiocephalic AV fistula at the wrist was initially described by Brescia and Cimino in 1966 as a side-to-side anastomosis, but an end-to-side configuration is preferred by most to reduce the risk of venous hypertension in the radial aspect of the hand.
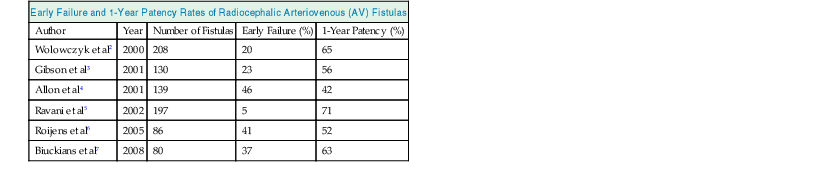
The primary patency of radiocephalic fistulas varies from center to center, but recent publications report high primary failure rates varying from 5% to 41% and 1-year primary patency rates of 52% to 71% (Table 91-1).2–7 Early thrombosis and nonmaturation of an AV fistula in the older comorbid population, who have poor upper limb vessels, are the major causes of these high primary failure and low patency rates. The patency of radiocephalic AV fistulas is poorer in women, so a proximal AV fistula might be preferable if the cephalic vein or radial artery is small.
Table 91-1
Early failure and 1-year patency rates of radiocephalic AV fistulas.
| Early Failure and 1-Year Patency Rates of Radiocephalic Arteriovenous (AV) Fistulas | ||||
| Author | Year | Number of Fistulas | Early Failure (%) | 1-Year Patency (%) |
| Wolowczyk et al2 | 2000 | 208 | 20 | 65 |
| Gibson et al3 | 2001 | 130 | 23 | 56 |
| Allon et al4 | 2001 | 139 | 46 | 42 |
| Ravani et al5 | 2002 | 197 | 5 | 71 |
| Roijens et al6 | 2005 | 86 | 41 | 52 |
| Biuckians et al7 | 2008 | 80 | 37 | 63 |
Nonmaturation of Radiocephalic Arteriovenous Fistula
The autogenous radiocephalic AV fistula needs time to mature and for the vein to enlarge to a size at which it can be needled for dialysis. Usually 6 weeks for maturation is advised. Earlier cannulation can damage the thin veins. Nonmaturation rates vary from 25% to 33%. The essential components of a successful AV fistula are a sufficient vein diameter of 4 to 5 mm for needling and a high blood flow so that blood can be drawn from the fistula at 300 to 400 ml/min. In reality, this requires a fistula flow of about 600 ml/min to prevent excessive recirculation and to permit adequate dialysis within the usual 4-hour time frame of HD treatment. Fistulas that fail immediately are the consequence of poor selection of vessels or poor technique. Regular duplex ultrasound investigation early after AV fistula formation, especially in fistulas that are not maturing, can detect poor flow, stenosis, and accessory branches, guiding the interventional radiologist and surgeon to the appropriate treatment.
Secondary Autologous Vascular Access
Although a primary radiocephalic AV fistula is preferable, the first-choice procedure is increasingly an upper arm AV fistula with use of an autogenous superficially located arm vein, especially in the dialysis population with associated comorbidities such as diabetes mellitus, coronary heart disease, and peripheral arterial occlusive disease.1
The upper limb is preferred to the lower limb for vascular access because of the ease of cannulation, comfort for the patient, and considerably lower incidence of complications. Similarly, autogenous conduits are preferable to the use of prosthetic grafts because of improved patency and lower risk of infection.
Forearm Cephalic and Basilic Vein Transposition and Elevation
Vein transposition or elevation increases the possibilities for creating a forearm fistula. The cephalic vein is preferred, but if it is unsuitable, the more deeply located basilic vein can be transposed from the ulnar to the radial side along a straight subcutaneous course from the elbow to the radial artery. Alternatively, a basilic vein–to–ulnar artery anastomosis can be performed with additional volar transposition to facilitate needling for dialysis.
Different surgical techniques, with or without transposition, have been advocated according to the forearm artery and vein location. In one study,8 91% fistula maturation was achieved with a range of techniques; 15% were suitable for a straightforward AV fistula, 33% required vein transposition from dorsal to volar for anastomosis to the appropriate artery, and the remaining 52% required superficial transposition of a vein on the volar aspect of the forearm before arterial anastomosis. Primary patency rates were 84% at 1 year and 69% at 2 years.
Needle cannulation may be difficult, particularly in obese patients. A forearm cephalic vein that is too deeply located may be made accessible for cannulation by transposition or elevation. In one study, the elevation technique was applied in obese patients with radiocephalic AV fistulas and cannulation difficulties; the primary failure rate was 15%, with a 1-year patency rate of 84%. After operation, all patients could be successfully cannulated for dialysis.9
Recently surgical lipectomy or liposuction has been advocated to facilitate cannulation of deeply located fistulas.10
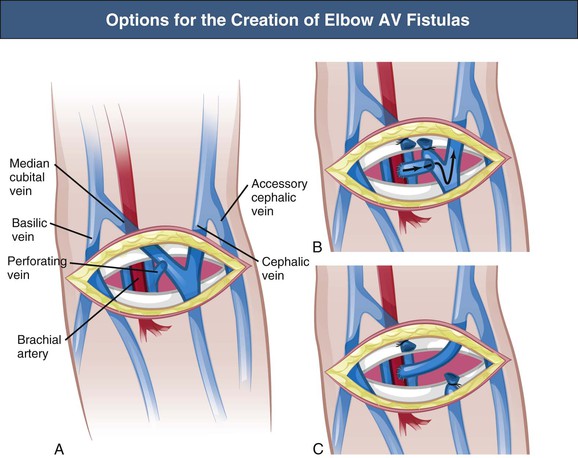
Elbow and Upper Arm Cephalic Vein Arteriovenous Fistula
The brachiocephalic and antecubital fistulas are two possible AV anastomoses in the elbow region. In addition, anastomosis between the transposed cephalic vein and brachial artery 2 cm proximal to the elbow may be executed, which provides an optimal situation for cannulation along the cephalic vein (Fig. 91-2). The outcome of the brachiocephalic AV fistula is usually good, with a high primary function rate and good long-term patency; studies showed a 10% early failure rate caused by nonmaturation and an 80% 1-year patency rate.11,12 Two-year primary, assisted primary, and secondary patency rates were 40%, 59%, and 67%, respectively. (Primary patency is functioning access without any intervention; assisted primary patency is functioning access after preemptive intervention for flow decline; secondary patency is functioning access after intervention for thrombosis.) Predictors of failure include diabetes mellitus and a history of contralateral forearm AV graft (indicating poor vessels). Therefore the primary patency of brachiocephalic fistulas is comparable to that of radiocephalic fistulas. The early failure and 1-year patency rates of brachiocephalic AV fistulas are shown in Table 91-2.11–15
Table 91-2
Early failure and 1-year patency rates of brachiocephalic AV fistulas.
| Early Failure and 1-Year Patency Rates of Brachiocephalic Arteriovenous (AV) Fistulas | ||||
| Author | Year | Number of Fistulas | Early Failure (%) | 1-Year Patency (%) |
| Murphy et al14 | 2002 | 208 | 16 | 75 |
| Zeebregts et al12 | 2005 | 100 | 11 | 79 |
| Lok et al11 | 2005 | 186 | 9 | 78 |
| Woo et al15 | 2007 | 71 | 12 | 66 |
| Koksoy et al13 | 2009 | 50 | 10 | 87 |

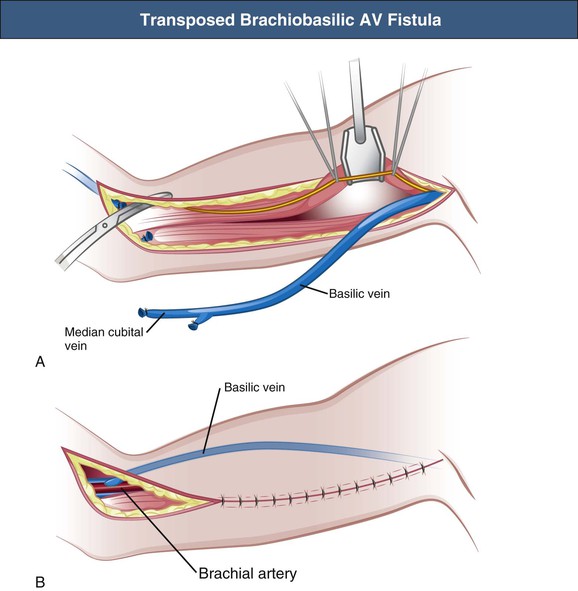
Upper Arm Basilic Vein Arteriovenous Fistula
The upper arm basilic vein is usually inaccessible for dialysis cannulation because of its medial and deep position. Therefore the basilic vein needs to be superficialized and transposed to an anterolateral position. The original technique of brachiobasilic AV fistula construction is a two-step approach. First, a brachiobasilic anastomosis is constructed, and in the second operation, usually after 6 weeks, the arterialized vein is mobilized into a subcutaneous position, becoming accessible for needling (Fig. 91-3); at the present time the brachiobasilic AV fistula creation may be performed as a one-stage surgical procedure, with elevation or transposition of the vein to a subcutaneous and anterolateral position at the time of creation of the AV anastomosis. A nonrandomized study comparing the different techniques of brachiobasilic AV fistula creation reported 86% to 90% 1-year patencies in all groups, with only 5% to 7% nonmaturation rates.16 Primary failure rates of 2% to 23% with 1-year patencies varying from 55% to 89% have been reported (Table 91-3).13,17–20 In comparison with brachiocephalic fistulas, brachiobasilic AV fistulas are more likely to mature, although they are more susceptible to late thrombosis. However, a randomized study showed similar patencies of brachiocephalic and brachiobasilic AV fistulas.13
Table 91-3
Early failure and 1-year patency rates of brachiobasilic AV fistulas.
| Early Failure and 1-Year Patency Rates of Brachiobasilic Arteriovenous (AV) Fistulas | ||||
| Author | Year | Number of Fistulas | Early Failure (%) | 1-Year Patency (%) |
| Segal et al17 | 2003 | 99 | 23 | 64 |
| Wolford et al18 | 2005 | 100 | 20 | 47 |
| Harper et al19 | 2008 | 168 | 23 | 66 |
| Keuter et al20 | 2008 | 52 | 2 | 89 |
| Koksoy et al13 | 2009 | 50 | 4 | 86 |

The technique of subcutaneous placement of the basilic vein has several advantages over forearm or upper arm graft implantation, with less infection and thrombosis. A meta-analysis comparing brachiobasilic AV fistulas with prosthetic grafts has shown superiority of the brachiobasilic AV fistula in primary and secondary patency rates, and it should therefore be used early in difficult access cases before the use of prosthetic grafts.21
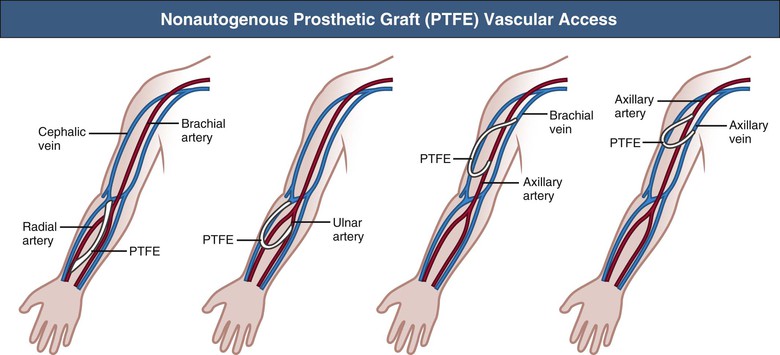
Nonautogenous Prosthetic Vascular Access
When autogenous AV fistula creation is impossible or the fistulas have failed, graft implantation should be considered as a vascular access conduit. Xenografts such as the ovine sheep graft (Omniflow) are popular materials as an alternative access conduit, with acceptable patency and low infection rates. The most frequently used implants are prosthetic grafts made of either polyurethane (Vectra) or polytetrafluoroethylene (PTFE). These prosthetic grafts can be implanted in a wide variety of locations and configurations in the upper limb (Fig. 91-4). At present, early cannulation of PTFE grafts (within 24 hours of surgery) is feasible because of newer graft compositions. Short-term functional patency is usually good, but stenosis (mostly at the graft-vein anastomosis) may lead to thrombotic occlusion within 12 to 24 months. The primary patency rates of prosthetic AV grafts vary from 60% to 80% at 1 year and from 30% to 40% at 2 years of follow-up. Secondary patency ranges from 70% to 90% and from 50% to 70% at 1 and 2 years, respectively.22–25 Intimal hyperplasia, with smooth muscle cell migration and proliferation and matrix deposition, is the major cause of stenosis formation and thrombosis. The cause of the intimal hyperplasia is uncertain, although the high wall shear stress, caused by the access flow, may denude the endothelial cell layer, resulting in platelet adhesion and initiation of a cascade of proteins that stimulate the smooth muscle cells to proliferate and to migrate.
Measures to Improve Graft Patency
Numerous experimental and clinical studies have defined the influence of graft material and graft design on AV graft patency. Modulating the geometry of the arterial inlet or venous outlet of the graft may have a beneficial effect on intimal hyperplasia. Clinical studies using tapered (at the arterial side of the graft) grafts did not improve patency rates, nor did cuff implantation at the venous anastomosis.26,27 However, primary patency did improve with the use of a cuff-shaped prosthesis (Venaflo).28 Grafts such as polyurethane, which are more distensible, could in principle influence intimal hyperplasia by the better matching of the stiff prosthesis with the compliant vein at the anastomotic site; however, in clinical studies, this feature was not of proven benefit.29
Pharmacologic Approaches for Access Patency
Aspirin, ticlopidine, and dipyridamole have some beneficial effect in maintaining patency of AV fistulas and grafts but increase the risk of hemorrhage.30 Clopidogrel may also be effective in reducing thrombosis of AV grafts and fistulas. Warfarin reduces AV graft thrombosis but increases the risk of hemorrhage.31 A large trial showed that dipyridamole plus aspirin had a significant but modest effect in reducing the risk of stenosis and improving the duration of primary unassisted patency of newly created AV grafts.32 In a large randomized study, clopidogrel improved primary radiocephalic fistula function but not maturation.33 Given the available evidence, antiplatelet agents should be used routinely in patients with AV grafts but not fistula. There is no role for the routine use of warfarin (coumadin), particularly in view of the recent evidence of adverse effects of warfarin in HD patients.
There have been suggestions that other drugs, such as calcium channel blockers and angiotensin-converting enzyme inhibitors, might be associated with improved AV fistula patency, but this requires confirmation with randomized studies.34 Fish oil reduced AV graft thrombosis in one randomized trial.35
Efforts have been made to inhibit the development of intimal hyperplasia pharmacologically with the cytotoxic agent paclitaxel. Paclitaxel wraps have been shown to reduce prosthetic graft intimal hyperplasia in animal models but have yet to be clinically evaluated.
Lower Limb Vascular Access
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree