Jeremy Levy
Plasma Exchange
The place of plasma exchange (plasmapheresis) in the management of renal disease has become clearer in the last 10 years, with increasing numbers of controlled studies comparing therapeutic plasma exchange with other treatments. Despite this, in many renal diseases the quality of published data still remains poor, and the precise role for plasma exchange unclear. Plasma exchange came into widespread clinical use after early reports of beneficial effects in Goodpasture disease in the mid-1970s. It is used to remove many large-molecular-weight substances from plasma, including pathogenic antibodies, cryoglobulins, and lipoproteins. Newer techniques have also been developed to allow more selective removal of plasma components, such as double-filtration plasma exchange, cryofiltration, and immunoadsorption with or without immobilized ligands.
Techniques
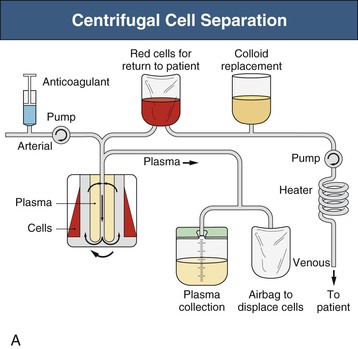
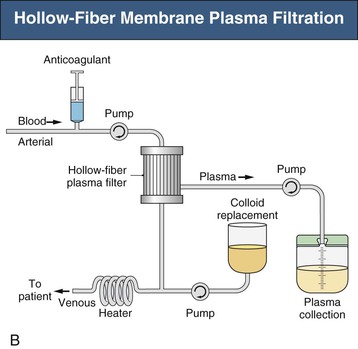
Plasma exchange can be carried out either by centrifugal cell separators or, more commonly in renal units, with hollow-fiber plasma filters and standard hemodialysis equipment (Figs. 99-1 and 99-2). Centrifugal devices allow withdrawal of plasma from a bowl with either synchronous or intermittent return of blood cells to the patient.1 There is no upper limit to the molecular weight of proteins removed by this method. The bowls and circuits are single use and disposable and require relatively low blood flow rates (90 to 150 ml/min). Platelet loss can be a particular problem with centrifugal devices, and platelet counts can decrease by as much as 50%. Membrane plasma filtration uses highly permeable hollow fibers with membrane pores 0.2 to 0.5 µm. Plasma readily passes through the membrane while the cells are simultaneously returned to the patient. All immunoglobulins will cross the membrane (IgG more efficiently than IgM); however, some large immune complexes and cryoglobulins may not be adequately cleared, although many membranes allow for clearance of molecules up to 3 million daltons. There is no loss of platelets, but hemolysis can occur if transmembrane pressures are too high (a rare complication). Blood flow rates required are higher (100 to 300 ml/min); there is no increase in rate of plasma filtration at higher blood flows, but an increased risk of hemolysis. Membranes used in plasma filters are polysulfone, polypropylene, cellulose diacetate, polymethylmethacrylate, or polyacrylonitrile.1 It has been suggested that the adsorptive properties of the membrane for cytokines and other biomolecules may account for some of the beneficial effects of plasma filtration. There have been occasional reports of mild adverse reactions in patients taking angiotensin-converting enzyme (ACE) inhibitors when plasma is filtered with ethylene vinyl alcohol or acrylic copolymer membranes. Reuse of plasma filters is not advised, but performance data do not indicate a major loss of function during routine plasma exchange. For patients with severe renal failure, sequential hemodialysis and plasma exchange can easily be performed with plasmafiltration.
Vascular access is usually achieved using standard central venous catheters, but if a patient already has an arteriovenous (AV) fistula, then of course this can be used. However, sometimes it is also possible to perform plasma exchange using peripheral access through large-bore, short, intravenous cannulas, placed in the antecubital fossa, especially with centrifugal devices because the blood flow rates required are low. Single-needle access using an AV fistula is also relatively easy to accommodate, especially for centrifugal plasma exchange, in which the blood removal and return can be asynchronous, but also for membrane filtration. Anticoagulation is almost always needed and must be carefully managed in patients with a bleeding risk, for example, those with thrombotic microangiopathy (TMA), recent or ongoing pulmonary hemorrhage, or a recent renal biopsy. In general, citrate is used for centrifugal plasma exchange, and heparin for membrane plasma filtration; however, citrate has particular advantages in patients at higher bleeding risk in view of its lack of systemic anticoagulant actions.1 When heparin is used, higher doses may be needed than in hemodialysis as a result of increased losses during the procedure (heparin is protein bound). Bolus doses of unfractionated heparin of 2000 to 5000 U are given initially, and then 500 to 2000 U/hr. Anticoagulant is administered prefilter.
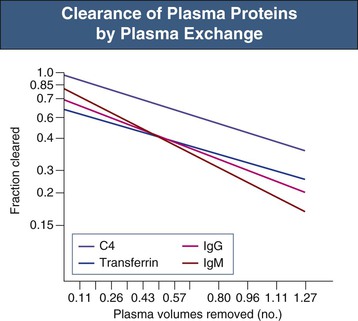
Both methods of plasma exchange require large volumes of colloid replacement. A single plasma volume exchange will lower plasma macromolecule levels by approximately 60%, and five exchanges over 5 to 10 days will clear 90% of the total body immunoglobulin (Fig. 99-3).1,2 For most patients, this is achieved by removing 50 ml of plasma per kilogram of body weight at each procedure (approximately 4 liters for a 75-kg person). Daily plasma exchange is likely to be most effective in rapidly depleting total body load in view of redistribution of immunoglobulins from the extravascular compartments, but there is no good evidence that intensity of exchanges has a major effect on outcomes except in patients with hemolytic uremic syndrome (HUS) with poor prognostic markers (see later discussion). Indeed, alternate-day exchanges are of proven efficacy in antineutrophil cytoplasmic antibody (ANCA)–associated diseases. Replacement with crystalloid is contraindicated because of the need to maintain colloid oncotic pressure. Synthetic gelatin–based plasma expanders or hydroxyethyl starch (Hespan) can be used as part of a replacement regimen but have been reported to cause a coagulopathy in patients with sepsis, and have a shorter half-life than human albumin, which is the mainstay replacement fluid. The major disadvantage of albumin solutions is the lack of clotting factors, with the potential development of depletion coagulopathy after plasma exchange. Fresh-frozen plasma (FFP) should be given, usually in addition to human albumin solution, in patients at particular risk of bleeding. If partial replacement is with FFP this should be given late during the exchange so that the constituents are not removed by the ongoing plasma exchange. However, almost all the serious complications of plasma exchange (hypotension, anaphylaxis, citrate-induced paresthesia, urticaria) have been reported in patients receiving FFP rather than albumin (see later discussion).1,3 Both human products carry a tiny risk of transmission of infectious diseases, especially viral. Standard regimens for plasma exchange are summarized in Table 99-1. Human albumin solution (4% to 5%) should be used for all exchanges except in thrombotic microangiopathies (in which plasma should provide the total exchange), and FFP should form part of the exchange when bleeding risk is high (ongoing pulmonary hemorrhage or within 48 hours of biopsy or surgery). If fibrinogen levels decrease to below 1.25 to 1.5 g/l or prothrombin time is increased 2 to 3 seconds above normal, then FFP should be administered.
Table 99-1
Practical regimens for plasma exchange in renal disease.
FSGS, Focal segmental glomerulosclerosis; HCV, hepatitis C virus; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
| Regimens for Plasma Exchange in Renal Disease | |||||
| Indication | Anti–Glomerular Basement Membrane Antibody (anti-GBM) Disease | Small-Vessel Vasculitis | Cryoglobulinemia | Recurrent FSGS After Transplantation | HUS/TTP |
| Duration of treatment | Daily, at least 14 days until anti-GBM antibodies 20% of baseline | Daily, 7-10 days depending on clinical response | At least 7-10 days or until clinical response | Daily, at least 10 days initially, then continuing less frequently, often for months | Daily for 7-10 days or until platelet count 80-100 × 109/l (sometimes needed twice daily) |
| Exchange volume | 50 ml/kg each treatment | As for anti-GBM disease | As for anti-GBM disease | As for anti-GBM disease | As for anti-GBM disease |
| Replacement fluid | Human albumin 5% (unless bleeding risk) | As for anti-GBM disease | As for anti-GBM disease | As for anti-GBM disease | Fresh-frozen plasma (FFP) or cryo-poor FFP |
| Additions to replacement fluid | 20 ml 10% calcium gluconate (occasionally more), 3 ml 15% KCl if not dialysis dependent, heparin 5000-10,000 U (unless citrate anticoagulation) | As for anti-GBM disease | As for anti-GBM disease | As for anti-GBM disease | As for anti-GBM disease (may need more calcium because of increased volume of FFP-containing citrate) |
| Immunosuppression | See Chapter 24 | See Chapter 25 | See Chapter 21 | See Chapter 29 | See Chapter 29 |
| Variations | FFP 5-8 ml/kg as part of exchange volume if hemorrhage risk (renal biopsy in last 48 hr, lung hemorrhage, platelets <40 × 109/l, fibrinogen <1.5 g/l); immunoadsorption may be as effective | As for anti-GBM disease Immunoadsorption may be as effective | As for anti-GBM disease | As for anti-GBM disease; may need to include replacement immunoglobulins if continuing long term; immunoadsorption may be as effective | |
Double-filtration plasma exchange (or cascade filtration) uses membrane filtration to separate cells from plasma, and then a secondary plasma filtration (pore size 0.01 to 0.03 µm) to remove plasma solutes based on molecular size. Most albumin is therefore returned to the patient, together with lower-molecular-weight proteins, reducing the need for replacement fluids. Cryofiltration uses a similar principle but exposes the filtrate to 4° C during the procedure, with the aim of precipitating cryoproteins. These techniques are not widely available.
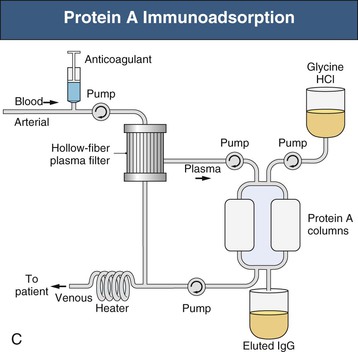
Selective and specific immunoadsorption techniques are increasingly available. Protein A immunoadsorption has been used to remove immunoglobulin alone from plasma, without the need for replacement fluids and without depletion of clotting factors and complement (see Fig. 99-1, C). Protein A selectively binds the Fc domains of immunoglobulin molecules, and the immunoadsorption columns can be repeatedly regenerated. Columns have been used for 1 year for a single patient on up to 30 occasions; however, the repeated acid stripping during regeneration does reduce the efficacy of antibody binding. This technique has been used to treat conditions in which autoantibodies are thought to play a key pathogenic role and usually in place of plasma exchange, such as in Goodpasture disease, rheumatoid arthritis, systemic lupus, or systemic vasculitis, and to remove anti-ABO or anti–human leukocyte antigen (HLA) antibodies in highly sensitized transplant recipients. In general, the reported efficacy has been equal to that of plasma exchange, although if used long term, immunoadsorption can be much more cost-effective in single patients because there is no requirement for replacement albumin or plasma. Specific ligands have also been immobilized onto columns for more specific removal of potentially pathogenic serum factors; ligands used include anti–human IgG, C1q, phenylalanine, hydrophobic amino acids, acetylcholine receptor, and β-adrenoreceptor peptides, and blood group–related oligosaccharides. Immunoadsorption of all varieties is not widely available because the initial column costs are high and few centers have much clinical experience. Costs of all other plasma exchange modalities are dominated by the costs of replacement albumin and plasma; equipment costs do not vary much among modalities, and all require skilled and trained nursing staff. Plasma filtration requires minimal extra support or training for dialysis nurses because the equipment is very similar to normal hemodialysis machines.
Complications
The complication rate of plasma exchange is not high.3 The Swedish registry reported no fatalities during 20,485 procedures, and an overall adverse incidence rate of only 4.3% of all exchanges (0.9% for severe adverse events) of which 27% were paresthesias, 19% transient hypotension, 13% urticaria, and 8% nausea.4 The Canadian apheresis registry has collected data on 144,432 apheresis procedures since 1981 and reported adverse events occurring in 12% of procedures (mostly minor), and overall in 40% of patients. Severe events occurred in only 0.4% of procedures. Three deaths were probably related directly to the procedure: one from a transfusion-related acute lung injury and two from complications from central venous catheters.5 An overall complication rate of 1.4% has been reported in more than 15,000 treatments in patients receiving albumin, and 20% in patients receiving FFP.1,3,5,6 Plasma exchange by centrifugation had a lower risk for adverse events than by filtration.
Other complications directly attributable to plasma exchange include citrate-induced hypocalcemia (presenting with perioral tingling and paresthesias) and citrate-induced metabolic alkalosis. Citrate is usually present in FFP (up to 14% by volume) or is administered in the extracorporeal circuit as an anticoagulant; it binds free calcium in plasma. Symptomatic hypocalcemia can be averted by infusing 10 to 20 ml of 10% calcium gluconate during each plasma exchange. Alkalosis is rare and is caused by metabolism of citrate to bicarbonate and failure to excrete the latter in patients with renal impairment.
Plasma exchange predictably increases the risk of bleeding as a result of depletion of coagulation factors in patients receiving albumin as sole replacement colloid. Prothrombin time is increased by 30%, and partial thromboplastin time by 100% after a single plasma volume exchange. Patients at risk of bleeding (pulmonary hemorrhage, postbiopsy, postoperative) should receive FFP (300 to 600 ml) with replacement fluids. Dilutional hypokalemia is avoided by adding potassium to the replacement albumin. An increased incidence of infection secondary to hypogammaglobulinemia has not been confirmed in recent series.3–6 Sepsis related to intravenous access is the most common infectious complication of plasma exchange. Hypotension can occur for a variety of reasons related to extracorporeal circuits, sepsis, and allergic reactions, but also if saline is used for exchanges with a concomitant reduction in serum oncotic pressure during the procedure. Cascade filtration can lead to hemolysis (in up to 20% patients) but rarely necessitates transfusion.
Mechanisms of Action
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree