Steven J. Chadban, Tony Kwan
Recurrent Disease in Kidney Transplantation
Kidney transplantation is a treatment, not a cure. Although transplantation may restore kidney function, it does not necessarily remove the cause of the original kidney disease. Glomerulonephritis (GN) and diabetes are the two leading causes of end-stage renal disease (ESRD) worldwide and are the primary underlying diseases of patients undergoing kidney transplantation, and both diseases may recur.
The longer that a transplant remains in situ, the more likely it is to be affected by recurrence. As graft survival rates have increased over the past 30 years, mostly because of more effective antirejection therapies that prevent early graft loss, the apparent incidence of recurrence has grown.1 An analysis of U.S. Renal Allograft Disease Registry data examining GN recurrence demonstrated a prevalence of 2.8% at 2 years, 9.8% at 5 years, and 18.5% at 8 years of follow-up after transplantation, and such patients were twice as likely to experience graft failure compared with those without recurrence.2
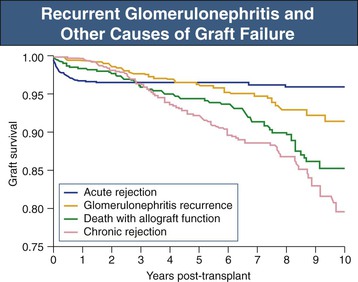
Recurrence has a powerful impact on transplant survival and one that is increasingly apparent with time after transplantation. In an analysis of data from the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) including more than 1500 patients with biopsy-proven GN who received a kidney transplant, biopsy-proven recurrence was found to cause graft loss in 0.5% within 1 year after transplantation, 3.7% within 5 years, and 8.4% within 10 years after transplantation3 (Fig. 108-1). This study and several others have found recurrent disease to be the third most common cause of graft failure beyond the first year after transplantation, behind death with a functioning graft and chronic allograft nephropathy (CAN), but substantially ahead of acute rejection3 (see Fig. 108-1).
De novo GN and new-onset diabetes after transplantation (NODAT) may also affect the transplanted kidney, although both are relatively uncommon. Both conditions may be difficult to distinguish from recurrence or from CAN. Like recurrent disease, the prevalence of both appears to increase with time after transplantation. Given the high incidence of NODAT coupled with general increases in graft survival,4 it is possible that de novo diabetic nephropathy in particular may become a significant clinical problem in the future. However, present data suggest that the major impact of NODAT in the first 10 years after transplantation is an increase in cardiovascular mortality with little impact on death-censored graft failure.5
Definitions
Diagnosis of recurrence requires histologic demonstration of the same disease involving both the native and transplanted kidneys. Diagnosis of recurrence causing graft failure requires a clinical decision that recurrence was the dominant contributor to graft loss (other contributors, such as CAN, may be present). A histologic diagnosis of the primary kidney disease is not obtained for all patients with ESRD, and the majority of transplant recipients do not undergo biopsy to look specifically for recurrent disease (which may require immunohistologic and electron microscopic examination of the biopsy specimen).1 Coupled with the tendency of clinicians to make a clinical diagnosis of CAN and thereby avoid biopsy in patients with declining graft function and proteinuria, the true incidence of disease recurrence is not known and is likely underestimated in existing literature.
Additional factors confound the available evidence. Many reports of disease recurrence are retrospective, single-center studies. Recall bias and incomplete documentation, changes in practice over time, and peculiarities of local patient populations and local practices may limit relevance to other populations. The most definitive reports have come from analyses of the large registry databases of Europe, the United States, and Australasia. Registries capture data on large numbers of patients but are subject to bias because of factors including unit participation rates; quantity and type of data collected; accuracy, uniformity, and consistency of reporting by units; and reliability of data entry. How recurrence is defined and diagnosed and which outcomes are measured is crucial. For example, IgA nephropathy (IgAN) recurred in 58% of patients in one series in which all recipients underwent biopsy,6 but in approximately 25% of cases in which biopsy was performed only when clinically indicated.7 When graft loss from IgAN recurrence is the outcome measure, the risk decreased to approximately 10% at 10 years of follow-up.3 Thus, definition of recurrence, outcome measures, study design, era, and source of data all need to be considered in assessing the published literature.
Recurrent Glomerulonephritis
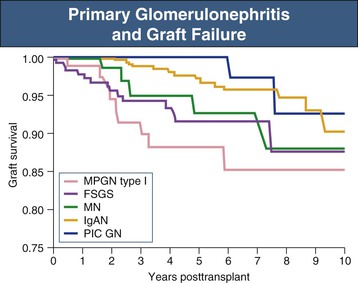
Virtually all GN may recur after transplantation; however, the rate and consequences of recurrence vary enormously. For example, anti–glomerular basement membrane (GBM) disease (Goodpasture disease) recurs only rarely, but when it does, it is likely to cause rapid graft loss. In contrast, dense deposit disease (DDD; formerly known as type II membranoproliferative glomerulonephritis [MPGN]) recurs in more than 80% of patients; however, the disease tends to be very slowly progressive, and graft survival beyond 10 years is typical. Numerically, recurrence of focal segmental glomerulosclerosis (FSGS), IgAN, and membranous nephropathy (MN) are the most frequently encountered clinical problems (Fig. 108-2).
Several factors may influence the risk of recurrence in addition to the underlying type of GN. Time of follow-up is clearly important and may be related to the duration of exposure of the graft to the nephritogenic factors responsible for GN.1 In general, grafts that are lost early because of rejection are exposed relatively briefly and rarely develop recurrence. In contrast, those grafts that survive long term are exposed to nephritogenic factors for longer and are more likely to develop recurrent GN. Consistent with this concept, recipients of human leukocyte antigen (HLA)–identical transplants rarely suffer rejection and enjoy prolonged graft survival but have a high rate of recurrent GN.8 In one report of HLA-identical recipients, recurrent GN was present in 36% to 42% of those in whom biopsy was performed and resulted in 24% of graft losses, being the second most frequent cause of graft loss after death in this group.8 Patients sustaining first graft loss from recurrent GN are also at higher risk of recurrence in a subsequent graft.
The type of immunosuppressive agents may be significant; however, data in this area are sparse. Given that immune mechanisms contribute to the pathogenesis of most types of GN and that immunosuppression is effective in the treatment of several types, it is logical that immunosuppression should decrease the incidence and severity of recurrence after transplantation. Improvements in immunosuppression over the past 40 years have led to reduced rates of acute rejection. Such improvements may also have also had an impact on rates of graft loss from GN recurrence, which have declined by nearly 50% over the past 10 years according to one ANZDATA registry analysis.9
United States Renal Data System (USRDS) registry data failed to demonstrate superiority of any individual immunosuppressive agent over others on the incidence of recurrence.10 Nevertheless, there are reports that suggest the immunosuppressive regimen may be important. For example, one small retrospective study found that induction therapy with rabbit antithymocyte globulin was associated with a lower risk of IgAN recurrence compared with either anti-CD25 antibodies or no induction.11 Corticosteroid withdrawal may also lead to increased rates of recurrence,12 especially in patients with underlying IgAN, in whom a twofold increase in the risk of graft failure from recurrence of IgAN has been reported.9 Similarly, inclusion or addition of cyclophosphamide to immunosuppressive regimens may be helpful in the treatment of recurrent vasculitis affecting the graft.13 The use of sirolimus may also result in proteinuria and renal dysfunction, especially in patients with an underlying GN, suggesting that sirolimus may accelerate glomerular injury in the graft.14 Effects of individual agents are likely to be disease specific, and much needs to be learned in this area.
Strategies to reduce the risk of recurrence have been reported. Bilateral native nephrectomy as a means of eliminating persistent antigenic stimulation appears unhelpful; indeed, nephrectomized patients experienced a higher incidence of recurrence compared with those with native kidneys left in situ in one large single-center, retrospective study.15 Induction of disease remission before transplantation and prolonged time on dialysis pretransplantation, both aimed at permitting disease “burnout,” do not appear to be effective except in the case of anti-GBM disease, in which a delay in transplantation until the patient has been serologically negative for at least 6 months virtually eliminates the risk of recurrence.3 Avoidance of living related donation has been debated for years but overall appears not to have any impact on risk of recurrence.16
As with GN in native kidneys, proteinuria, hematuria, and deterioration in kidney function are the cardinal manifestations of recurrent GN. The pattern of renal and extrarenal manifestations is frequently similar to that of the native disease, except that, in our opinion, the rate of progression may in general be slower. Extrarenal features of the primary condition may recur, such as thrombocytopenia and hemolysis in hemolytic uremic syndrome (HUS) and extrarenal vasculitis in recurrent antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis. Serology may be helpful in some patients, such as anti-GBM antibody detection in those with Goodpasture disease, but may be inconsistent in others, such as those with recurrent lupus nephritis (LN).
The differential diagnosis of recurrent GN is clinically important because management may vary according to the diagnosis (Table 108-1). CAN and diabetic nephropathy, recurrent or de novo, may both present with progressive graft dysfunction, proteinuria, and hypertension and may therefore be clinically indistinguishable from recurrence. De novo GN should also be considered. Viral diseases of the kidney, particularly BK nephropathy, are an important alternative to consider because a reduction in immunosuppression and specific antiviral therapy may provide benefit. The need to exclude obstructive uropathy and tumors involving the graft warrants an ultrasound scan. Finally, recurrence may coexist with CAN or calcineurin inhibitor toxicity. Indeed, every condition that can lead to chronic graft dysfunction should be considered in the differential diagnosis of recurrence (see Table 108-1 and Chapter 107).
Table 108-1
Differential diagnosis of recurrent glomerulonephritis.
EBV, Epstein-Barr virus; PCR, polymerase chain reaction; PTLD, post-transplantation lymphoproliferative disease.
| Differential Diagnosis of Recurrent Glomerulonephritis | |||||
| Diagnosis | Frequency and Timing | Clinical Features | Lab Features | Biopsy Features | Management |
| Recurrent glomerulonephritis | Common; variable timing, days to years | Proteinuria, hematuria, renal impairment, hypertension | Similar to primary glomerulonephritis; serology may be negative | Same as primary glomerulonephritis4,5 | Disease specific |
| De novo glomerulonephritis | Uncommon; variable timing but typically later than recurrence | Proteinuria, hematuria, renal impairment | Type specific | Type specific4,5,8 | Antiprogression strategies (see Chapter 73) |
| Chronic allograft nephropathy | Very common; increasing incidence with time | Hypertension, proteinuria, renal impairment Calcineurin inhibitor exposure | Tubulointerstitial fibrosis, arteriolar hyalinosis, transplant glomerulopathy | Minimize calcineurin inhibitor and antiprogression strategies (see Chapter 73) | |
| Graft pyelonephritis | Uncommon Typically early after transplantation | Fever, pyuria, renal impairment | Positive blood or urine cultures | Neutrophil infiltration | Antibiotics |
| BK nephropathy | Uncommon; typically 1-5 yr after transplantation | Renal impairment, decoy cells in urine | Serum BK PCR positive | Tubulitis with tubular cell atypia and inclusions, normal glomeruli | Minimize immunosuppression and consider antiviral drugs |
| Acute rejection | Common; early | Renal impairment, oliguria | Nonspecific | Tubulitis with or without vasculitis15 | Increase immunosuppression |
| Renal tumor/PTLD | Uncommon, rare; early or late | Renal impairment, renal mass | Anemia, EBV positive | Atypical cells, mitoses, monoclonality | Minimize immunosuppression, consider chemotherapy |
Histologic evidence of recurrence is required in all patients. Biopsy can provide the diagnosis, exclude alternative diagnoses that may require different approaches to treatment, and provide important prognostic information pertinent to the affected graft and also relevant to any future consideration of retransplantation. Full evaluation of the biopsy specimen by light microscopy, immunohistology, and electron microscopy is desirable and in many patients essential to confirm recurrence.17 Light microscopy and immunohistology are necessary to differentiate recurrent from de novo GN, rejection, and calcineurin inhibitor toxicity. The presence of tubulitis should suggest acute rejection. CAN may produce chronic interstitial inflammation and transplant glomerulopathy, which may be indistinguishable from MPGN on light microscopy (Fig. 108-3; see also Chapter 107). The use of immunohistology to define the immunoglobulin and complement component content of immune deposits and electron microscopy to establish the structure of basement membrane and location of deposits may clarify the diagnosis.17 Histologic appearances are typically no different from the patterns of GN in native kidneys and are illustrated in the relevant chapters of Section IV. The risk of recurrence in common patterns of renal disease is summarized in Table 108-2.
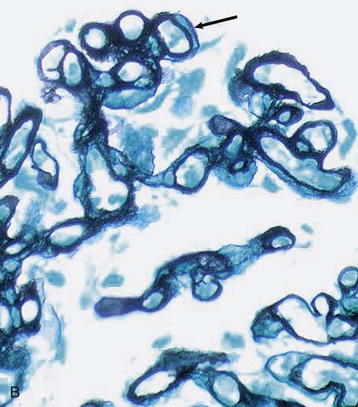
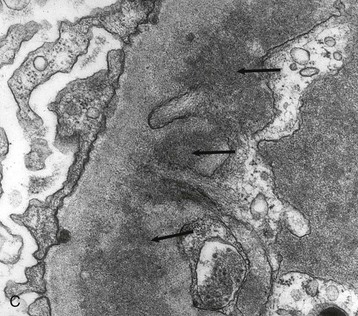
Table 108-2
Recurrent diseases in renal transplants and effects on graft survival.
GBM, Glomerular basement membrane.
| Recurrent Diseases in Renal Transplants and Effects on Graft Survival | ||
| Disease | Clinical Recurrence Rate (%) | Graft Loss in Recurrent Disease (%) |
| Primary focal segmental glomerulosclerosis | 20-50 (children), 10-15 (adults) | 40-50 |
| Membranoproliferative glomerulonephritis Type 1 | 20-30 | 30-40 |
| Dense deposit disease | 80 | 20, often late |
| Hemolytic uremic syndrome (HUS) | ||
| Classical D+ HUS | 0-13 | Uncommon |
| Atypical D− HUS | 30-50 | 55-100 |
| Familial HUS | 57 | Approaching 100 |
| IgA nephropathy | 30-40, increases with longer duration of follow-up (30%-60% histologic recurrence rate) | 16-33 |
| Henoch-Schönlein purpura | Rare (despite 50% histologic recurrence rate) | Rare |
| Membranous nephropathy | 10-29 (histologic recurrence may be more common) | Up to 50 |
| Systemic vasculitis, including Wegener granulomatosis and microscopic polyangiitis | 10-20 | 20-50 |
| Anti-GBM disease (Goodpasture disease) | <5 | 50 |
| Systemic lupus erythematosus | 1-30 | Rare |
| Amyloidosis | 25 | 10-20 |
Recurrence of Specific Glomerular Diseases
IgA Nephropathy and Henoch-Schönlein Purpura
IgA nephropathy is the most common form of GN leading to ESRD, and affected patients frequently become transplant recipients. Histologic recurrence is frequent and increases with time; one study in which all recipients were subjected to protocol biopsy found recurrence in 58%.6 Recurrence is difficult to predict. Patient characteristics, pretransplantation course, serum IgA glycosylation, and angiotensin-converting enzyme (ACE) genotype have been found to have no predictive value.1,6,7 Choice of immunosuppression after transplantation is controversial. One large USRDS registry analysis suggested that individual drug choices did not affect the risk of graft loss attributed to recurrence.10 In contrast, a recent retrospective analysis of ANZDATA including 1521 allograft recipients who underwent transplantation because of biopsy-proven GN found that continuation of corticosteroids was strongly associated with protection from graft loss caused by recurrence of IgAN, although not from other types of GN.9 Observational data also suggest that induction with antithymocyte globulin may afford relative protection.11 Living related donor transplantation has been associated with an increased risk of recurrence and graft loss in some series but not in others,3 and the avoidance of living related donor transplantation in patients with IgAN is not justified.
The clinical expression of disease is variable and time dependent. Graft loss within the first 3 years after transplantation is uncommon (see Fig. 108-2), although it can occur, particularly when IgAN in the native kidney was rapidly progressive or after previous graft loss resulting from recurrence.7 The longer-term outlook is not benign, because progressive graft loss over time has been documented by all major studies. In one registry study that included 587 patients with biopsy-proven IgAN, the risk of graft loss from IgAN recurrence was approximately 10% within 10 years after transplantation,3 although a follow-up study suggests that this risk may be halved in the current era, particularly when corticosteroids are used during the maintenance phase.9
Recurrence of Henoch-Schönlein purpura (HSP) nephritis is less well characterized but appears to be similar to IgAN in all respects. A recent analysis of the United Network for Organ Sharing (UNOS) database including 339 patients with ESRD caused by HSP who received a first kidney allograft reported a frequency of graft loss caused by recurrence of 13.6% and no difference in 10-year graft survival between recipients with IgAN (58.4%) or HSP (59.3%) as their primary renal disease.18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








