Chapter 96 USE OF BOWEL IN LOWER URINARY TRACT RECONSTRUCTION IN WOMEN
The use of small and large bowel for bladder augmentation or substitution has been reported by surgeons since the first experimental report by Tizzoni and Foggi in 1888.1 Bladder enlargement by augmentation enterocystoplasty is predominantly offered to women with neuropathic and non-neuropathic bladder dysfunction who have not responded to pharmacologic regimens. Female patients in whom complete removal of bladder and urethra is mandatory because of urologic or gynecologic malignancies and those who have a bladder problem and irreparable loss of the functional urethra are candidates for urinary diversion. The underlying disease and the patient’s preference must be considered when deciding on the type of urinary reconstruction or diversion, such as bladder augmentation or orthotopic bladder substitution to the trigone or bladder neck, orthotopic bladder substitution to the urethra, continent cutaneous urinary diversion, or continent anal urinary diversion.
HISTORY OF AUGMENTATION ENTEROCYSTOPLASTY AND ORTHOTOPIC BLADDER SUBSTITUTION
Augmentation enterocystoplasty is considered the last resort of functional bladder reconstruction before urinary diversion is required. Females with benign diseases such as intractable idiopathic or neurogenic detrusor overactivity incontinence, low-compliance bladder from irradiation, or interstitial cystitis may benefit from enlarging the organ with bowel segments or removing the diseased bladder tissue and substituting it with bowel segments.2–6
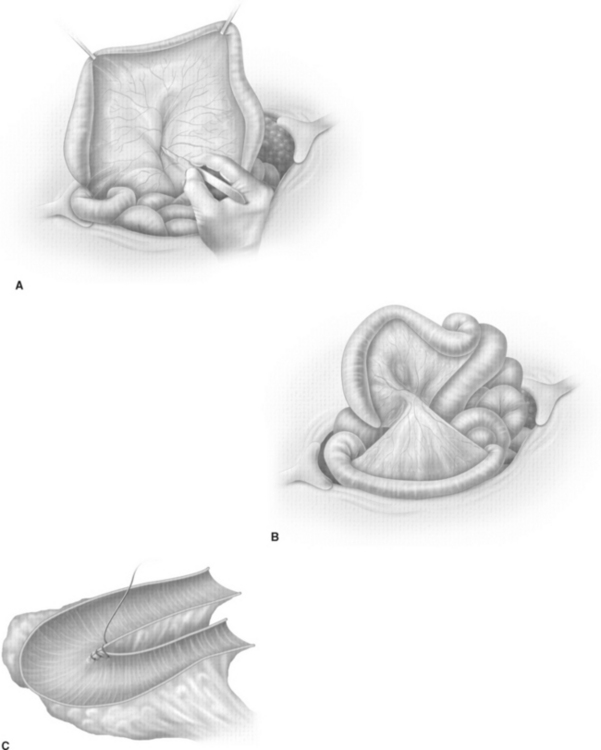
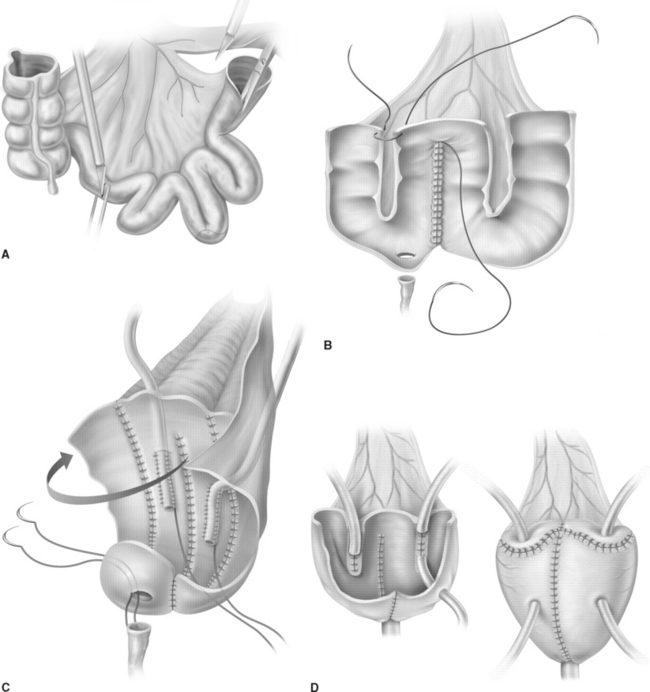
The cup-patch technique for bladder augmentation was first reported by Goodwin and colleagues for small bowel7 (Fig. 96-1) and for large bowel8 in 1959. A 20- to 25-cm ileal segment serving as the cup is opened at its antimesenteric border before it is formed to a U-shaped patch, folded over, and sutured to the bladder dome. When supratrigonal cystectomy is required (i.e., in interstitial cystitis or detrusor hyperreflexia), the resection line runs just above the trigone, leaving the ureteral orifices in place for preservation of the natural anti-reflux mechanism and avoiding potential complications of ureteral reimplantation into bowel.
Bladder substitution to the bladder neck may become necessary in female patients with interstitial cystitis because of inflammation of the remnant trigone and when preexisting ureteral reflux or obstruction is apparent. Bladder augmentation or substitution can be performed with different bowel segments, such as ileum only,9 ileum combined with cecum,10 or sigmoid colon only.11 An additional sling procedure or colposuspension may be required when coexisting stress incontinence is apparent.6
Flood and coworkers6 reported the long-term outcomes of 106 patients, including 82 women, who underwent augmentation enterocystoplasty. Preoperatively, all patients had reduced bladder compliance or detrusor overactivity caused by different underlying benign diseases. The overall success rate was 95% for all patients. Postoperative bladder capacity increased significantly from 108 to 438 mL. Urodynamic evaluation revealed stress incontinence in 15 patients (14%). Six of these patients had occasional leakage and did not require further treatment, and five were successfully treated by a sling procedure or collagen injection.
Complete relief of clinical symptoms of bladder pain and frequency was reported for 73% to 100% of patients in several series when enterocystoplasty was performed for intractable forms of interstitial cystitis.2–4 Between 66% and 86% of female patients were able to void spontaneously; the remainder had to empty their bladders by intermittent self-catheterization.
Linn and associates12 reported the clinical outcomes of 23 patients, including 22 women and 1 man, who underwent subtrigonal (n = 17) or supratrigonal (n = 6) cystectomy and orthotopic bladder substitution with the ileocecal pouch (i.e., Mainz pouch I) for interstitial cystitis. Postoperative bladder capacity was significantly increased, and urinary frequency was significantly decreased in all patients. Complete relief of clinical symptoms was achieved in 82% of the patients with subtrigonal cystectomy and in 100% of the patients with supratrigonal cystectomy. All patients who underwent supratrigonal cystectomy were able to void spontaneously, whereas 41% of the patients in whom the trigone was resected had to empty their bladder by intermittent self-catheterization.
ORTHOTOPIC BLADDER SUBSTITUTION
The first radical cystectomy in combination with an orthotopic bladder substitution for treatment of bladder cancer in a woman was reported by Tscholl in 1987,13 but before 1990, orthotopic urinary diversion was mostly limited to male patients when radical cystoprostatectomy was performed.14 Removal of the urethra was considered mandatory in female patients with bladder cancer to provide an adequate surgical margin. It was considered impossible to achieve urinary continence with the remaining short female urethra. Studies of cystectomy specimens that were removed for bladder cancer and a better anatomic understanding of the continence mechanism made it likely that urinary diversion to the urethra could be applied to selected female patients.15–17
Ileal Bladder Substitutes
In 1987, Ghoneim and colleagues18 described the urethral Kock pouch, which used 45 to 50 cm of the distal ileum.18 The proximal third of ileum is preserved for the reflux-preventing valve. The reservoir is created by the distal two thirds of bowel, which is opened along its antimesenteric border before folding it into a Ushape and performing a side-to-side anastomosis of the adjacent margins with a single layer of running suture. A nipple valve is created by isoperistaltic ileal intussusception and fixed by three or four rows of metal staples. Its position is secured by interrupted seromuscular sutures at the base of the nipple valve. The intestinal plate is folded over and closed by a single layer of running suture. The reservoir is rotated such that the posterior aspect is brought anteriorly and positioned into the pelvis with the apex facing toward the urethral stump, where the pouch is opened and anastomosed to the urethra by interrupted sutures. Ureterointestinal reimplantation is performed by end-to-side anastomosis of the ureters to the afferent limb of the nipple valve.
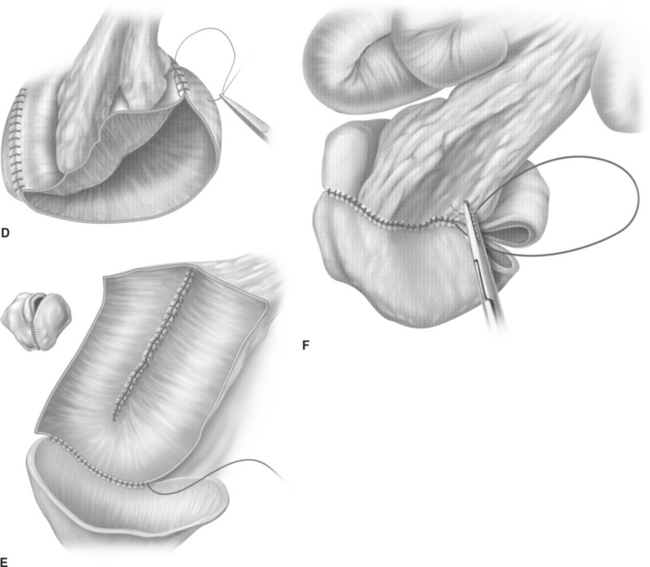
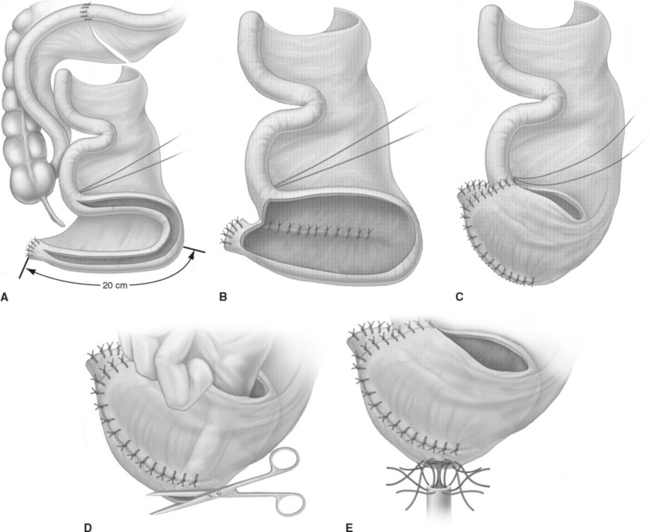
The T-pouch ileal neobladder was established in 1998 as a modification of the urethral Kock pouch diversion (Fig. 96-2).19 The afferent segment is created by an 8- to 10-cm segment of the proximal ileum. Its distal portion of 3 to 4 cm is tapered and tunneled into a serosa-lined tunnel, which is created at the midline anastomosis of the back wall of the pouch.
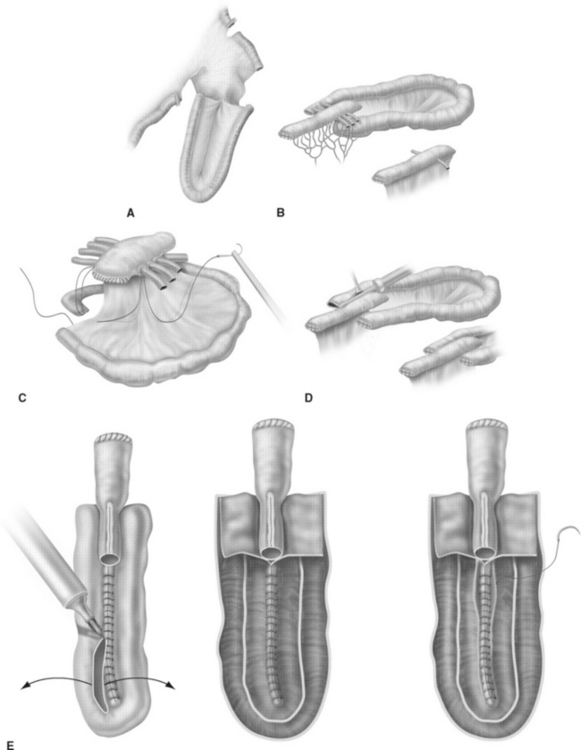
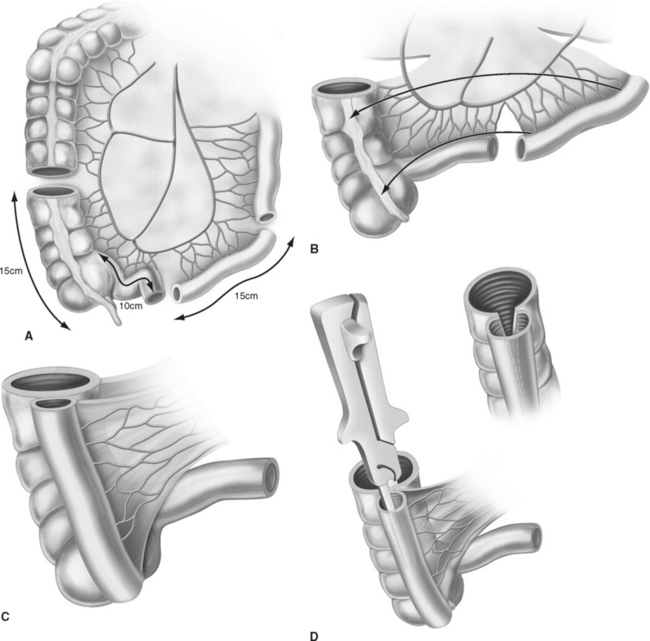
The ileal neobladder was established in 1987 by using a 60- to 70-cm ileal segment and leaving the distal portion of the terminal ileum and Bauhin’s valve intact (Fig. 96-3).20 The ileum is folded in a W-shaped manner and opened at its antimesenteric border except over a 5-cm segment, where the incision is made close to the anterior mesentery to create a posterior flap, which serves as the new bladder neck and to which the urethra is anastomosed. The reservoir is accomplished by side-to-side anastomosis of the posterior walls before the anterior wall is folded over and closed. Anti-refluxive ureteral reimplantation was initially favored using the LeDuc technique of creating a mucosal sulcus21 or by using the Abol-Enein technique of serosa-lined extramural tunnels.22 Since 1996, the technique of ureterointestinal implantation is predominantly performed by a refluxive ileoureteral end-to-side anastomosis into two 3- to 5-cm chimneys of ileum serving as afferent segments.23
Studer’s ileal neobladder was established in 1985 by using 60 to 65 cm of terminal ileum and leaving the proximal 25 cm intact for creation of an isoperistaltic afferent limb (Fig. 96-4).24 The distal 40 cm of ileum is folded into a U-shape and opened antimesenterically before the medial borders of the U are anastomosed to each other by a single layer of running suture. The ureterointestinal anastomoses are performed by direct end-to-side anastomosis to the proximal portion of the afferent limb. The reservoir is finalized by folding the bottom of the U over the proximal end. Before complete closure, an incision is placed into the most dependent part of the reservoir for the ileourethral anastomosis.
Ileocecal Bladder Substitute: Orthotopic Mainz Pouch
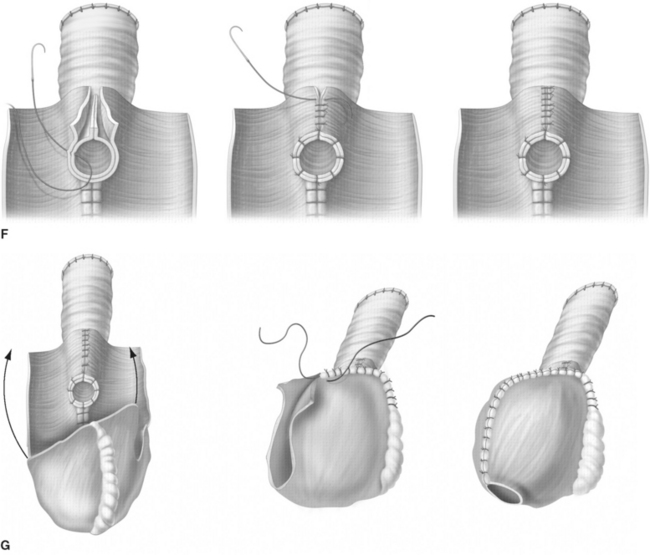
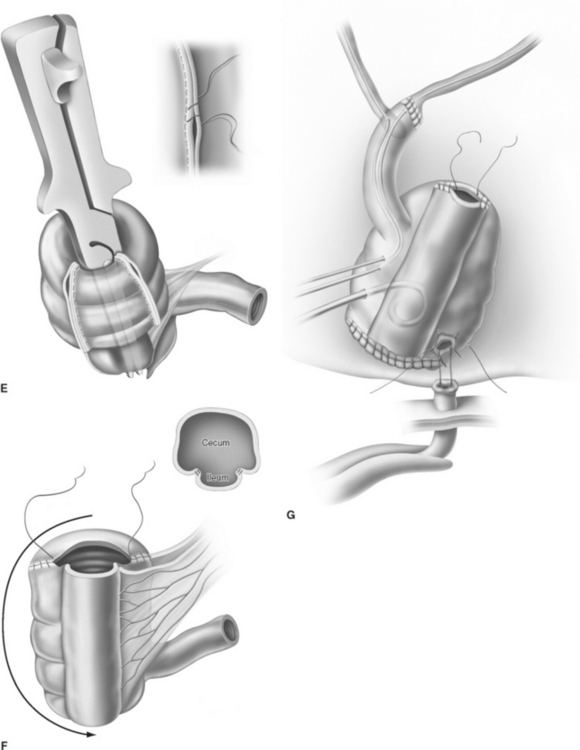
For creation of the orthotopic ileocecal reservoir (i.e., orthotopic Mainz pouch), a 10- to 15-cm segment of cecum in continuity with 20 to 30 cm of terminal ileum is isolated. The bowel is opened along its antimesenteric border. The opposing margins of the bowel are anastomosed to each other by a single row of running suture to create the posterior pouch wall. Originally, the ureters are implanted through a submucosal tunnel into the ascending colon to prevent reflux. Urethrointestinal anastomosis is performed by an incision at the base of the cecum. The reservoir is closed by side-to-side running sutures. In 2003, El-Mekresh and coworkers25 reported a modified technique of ureterointestinal reimplantation into the terminal ileum with the ileocecal valve serving as an anti-reflux-mechanism by using an end-to-end anastomosis (Wallace) for the left ureter and end-to-side anastomosis (Nesbit) for the right ureter with the afferent ileal limb (Fig. 96-5).
The rates of ileal neobladder–related early and late complications in a large series of 363 male patients were reported to be 15.4% and 23.4%, respectively.26 Early pouch-related complications included urinary leakage (7.7%), pyelonephritis (7.4%), and early obstruction of the ureteroileal anastomosis (3.0%). Late complications were associated with the ileoureteral anastomosis and upper urinary tract, including stenosis (9.3%), acute pyelone-phritis (6.3%), and reflux (3.3%). Ileourethral anastomotic strictures were seen in 2.2%, 96.1% of the patients were able to void spontaneously, and daytime continence was achieved in 95%.
Ghoneim and associates27 reported the clinical outcomes of 185 male patients treated with the urethral Kock pouch diversion. Deterioration of the upper urinary tract occurred in 13.7% of the renal units, with 9% due to reflux and 4.7% due to anastomotic strictures. Calculus formation was recognized in 18%, and urethral anastomotic strictures were encountered in 5.1%. Daytime and nighttime continence rates were 92% and 73%, respectively.
Perimenis and colleagues13 evaluated the long-term outcomes of male patients treated with Studer’s ileal neobladder with an afferent tubular segment. Nine (3.5%) of 254 renal units were treated for ureterointestinal anastomotic strictures. At the latest follow-up, renal parenchyma was normal in 246 units (97%), and serum creatinine levels remained unchanged compared with preoperative values in all patients. Daytime continence rates were 94% and 91% at 5 and 10 years, respectively, whereas nighttime continence rates were 72% and 60%, respectively. Residual urine volumes greater than 100 mL were recognized in 8% and were associated with urethrointestinal anastomotic strictures or protrusion of the intestinal mucosa into the urethra.
Cancrini and coworkers28 reported their 2-year experiences of orthotopic urinary diversion by Studer’s ileal neobladder in seven female patients.28 The rate of urinary continence was 100% during the day and 71% at night. Urodynamic studies 12 months postoperatively revealed a good neobladder capacity of 320 mL and no evidence of stress incontinence or significant levels of residual urine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree