Chapter 1 DEVELOPMENTAL ANATOMY AND UROGENITAL ABNORMALITIES
Knowledge of the prenatal development of the genitourinary system is essential to understand congenital disorders and normal urinary tract function and anatomy. This chapter summarizes the key milestones in genitourinary tract development at the organ and cellular levels. Many genes appear to play key roles in the molecular signals for development and differentiation of components of the genitourinary system. These genes are temporally and locally expressed during development, and without them, normal development fails.1 The kidney development database2 (http://www.ana.ed.ac.uk/anatomy/database/kidbase) provides a list of these genes, and updated or revised designations can be found in the international database (http://www.gene.ucl.ac.uk/nomenclature).
DEVELOPMENT OF THE GENITOURINARY SYSTEM
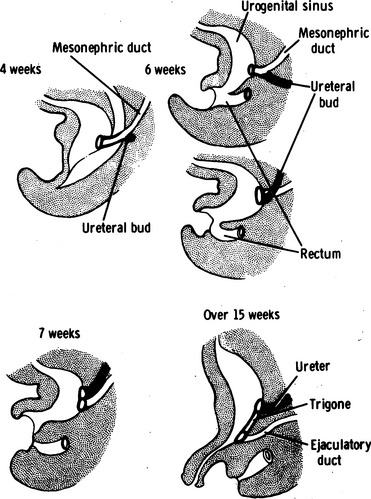
The cloaca ultimately is divided into the anterior urogenital sinus and the posterior rectum (Fig. 1-1), although the mechanism is debated. It was once believed that urorectal folds on either side of the midline grew caudomedially to fuse with the cloacal membrane and divide the cloacal membrane into the urogenital sinus and the dorsal rectum by week 7. Subsequent regression of the tail then rotated the urogenital sinus and rectum dorsally. Some investigators3,4 have suggested that the urorectal septum may not exist or may not fuse with the cloacal membrane.
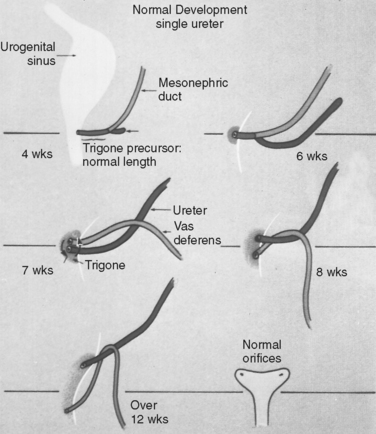
The development of the urinary tracts and portions of the genital system is induced by the mesonephric and paramesonephric (müllerian) ducts. Both ductal systems grow toward the urogenital sinus; the mesonephric ducts grow medially, whereas the müllerian ducts have already fused into a single midline structure. Fusion of the wolffian ducts with the cloaca occurs by the middle of the fourth week (day 24). The junction of the müllerian ducts and the urogenital sinus is a central embryologic location called Müller’s tubercle. The mesonephric ducts bend laterally; at this bend, a ureteric bud forms. The portion of the mesonephric duct between the urogenital sinus and the ureteric bud is called the common nephric duct, and by day 33, it is absorbed into the urogenital sinus, providing an island of mesoderm in the otherwise endoderm-based urogenital sinus (Fig. 1-2). This mesodermal island expands laterally to become the trigone of the bladder. The location of the ureteric bud relative to the urogenital sinus determines whether the ureteral orifices will be orthotopic; a ureteric bud that originates on a short, common nephric duct will be incorporated sooner into the bladder, with resultant lateral displacement of the ureteral orifices. The intramural ureteral tunnel predisposes to vesicoureteral reflux. Conversely, ureteric buds that are located a great distance from the urogenital sinus will be incorporated into the urogenital sinus later and may be associated with ectopic drainage into surrounding structures.
The ureteric bud continues to grow craniolaterally while the mesonephric duct (distal to the bifurcation of the common nephric duct into the mesonephric duct and ureteric bud) grows caudomedially. The ureter undergoes a process of obstruction during the sixth week (37 to 40 days) and then is recanalized from the central portion to the cranial and caudal limits. Incomplete recanalization at either end may account for obstruction at the ureteropelvic junction or at the ureterovesical junction, where a thin transient membrane (i.e., Chwalla’s membrane) may fail to dissolve. The cuboidal epithelium of the immature ureter evolves to a lining of transitional cells by 14 weeks.5
The urogenital sinus expands caudally to form the bladder and gives rise to the posterior urethra in males or the entire urethra and distal third of the vagina in females. The cranial portion of the urogenital sinus tapers during the third month of gestation so that the allantois forms the urachus and the saccular bladder remains in place. The intramural bladder wall develops throughout the remainder of gestation, with collagen formation beginning in the lamina propria and with subsequent intercalation between intramural muscle fibers. Intramural muscle fibers form as perivesical splanchnic mesoderm matures after induction by epithelial-mesenchymal interactions. Compliance of the fetal bladder increases over time in human fetuses and in animal models.6,7 Koo and colleagues7 showed a decreasing ratio of type 3 to type 1 collagen in the fetal bovine bladder; the changing ratios of perivesical collagen and muscle likely account for at least a portion of this evolution.
Renal and Ureteral Development
The metanephros gives rise to the fetal kidney. It forms in the sacral region as the ureteric buds arise from the mesonephric ducts. As the ureteric buds grow cranially, they encounter metanephric mesenchyme on about day 28. After the ureteric bud contacts the mesenchyme, release of many factors culminates in reciprocal induction of growth factors governing the development of the metanephric system. The ureteric bud divides repeatedly between weeks 6 and 32 of development, ultimately giving rise to the collecting system: the collecting ducts, calyces, renal pelvis, and ureter. The metanephric mesenchyme gives rise to the parenchymal portions of the kidney that perform filtration and clearance: the glomeruli, proximal and distal tubules, and loop of Henle. Because of the lengthy period during which branching of the ureteral bud occurs, the growth of the metanephric mesenchyme that will give rise to renal parenchyma is not uniform; nephrons at the juxtamedullary region are formed earlier and mature sooner than nephrons in more peripheral locations. Nephrons undergo four defined stages of development in the human. Stage I occurs when the metanephric mesenchyme is fully discrete from the ureteral bud. Stage II begins when the S-shaped nephron connects with the ureteral bud. In stage III, an ovoid structure emerges, and in stage IV, a round glomerulus is seen. Most nephrons in humans are stage IV at birth, although maturation is completed fully in the early postnatal period.5
As the kidneys grow, their location in the embryo becomes progressively more cranial; this is likely caused by active growth of the kidney parenchyma and by increased differential growth of the caudal portion of the embryo. As a result, the kidneys ascend from their initial pelvic location to the upper retroperitoneum. As renal ascent proceeds, new blood vessels are generated cranially, and the more caudal blood vessels break down. In postnatal patients with renal ectopia, the renal blood supply is typically anomalous because angiogenesis is arrested when renal ascent ceases. The possibility of an aberrant blood supply should be considered in any patient with renal ectopia.
Abnormalities of the Urogenital Sinus
Bladder exstrophy occurs in approximately 1 of 30,000 births and is seven times more likely in children conceived through in vitro fertilization.8 This disorder is characterized by early rupture of the cloacal membrane, which is sometimes related to an intrinsic defect in the membrane. It is more common in males than in females by a ratio of approximately 2:1 to 6:1,9 and it is related to epispadias and to cloacal exstrophy. The latter condition is also associated with early rupture of the cloacal membrane, although it occurs much less commonly (1 in 200,000 to 400,000 births10). Although no genes associated with either condition have been definitively identified, the risk of bladder exstrophy is substantially greater with an affected relative (1 in 275) or an affected parent (1 in 70).9 Mesenchymal ingrowth between the ecto-dermal and endodermal layers of the cloacal membrane ultimately results in formation of the lower anterior abdominal wall and division of the cloaca into the anterior urogenital sinus and posterior rectum. Both disorders are associated with malformations of other organ systems, including the limbs, lower anterior abdominal wall, pelvic girdle, and in the case of cloacal exstrophy, the hindgut. Management of these conditions remains challenging.
Gonadal Development
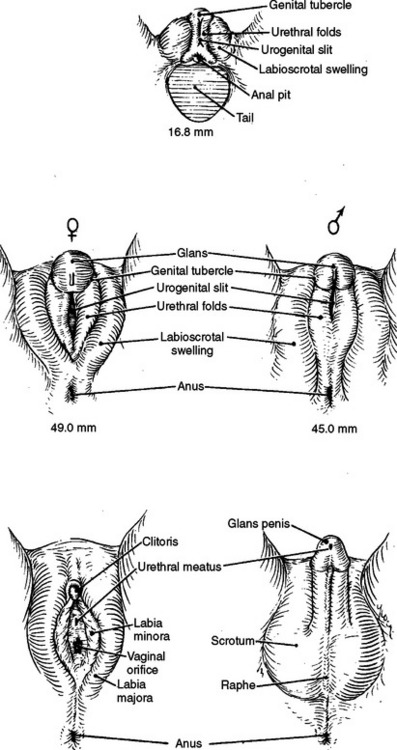
Development of the testes and ovaries is initiated in the fifth week of gestation, when germ cells from the yolk sac migrate to the posterior body wall, inducing formation of the urogenital ridge medial to the mesonephros (Fig. 1-3). Invasion of the adjacent mesenchyme in the sixth week creates a primitive gonad with epithelium and blastema; the latter is formed from loosened epithelial cells. Persistent growth of the germinal epithelium into the adjacent mesenchyme forms cords that ultimately branch many times and form seminiferous tubules.
Initially, all embryos have the potential to become male or female; the development of internal or external genitalia is an event influenced by genetic, endocrine, and paracrine factors. SRY, a gene on the short arm of the Y chromosome, induces formation of the Sertoli and Leydig cells. It also induces secretion of anti-müllerian hormone (AMH), formerly called müllerian-inhibiting substance (MIS), which induces regression of the müllerian system between 8 and 10 weeks’ gestation.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree