Sunjay Jain, James E. Dyer, Evangelos G. Gkougkousis, J. Kilian Mellon
Urologic Issues for the Nephrologist
Close interaction between nephrologists and urologists is crucial to the optimal management of a number of common clinical problems. A proper understanding of urologic strategies helps the nephrologist ensure that patients with these problems are given clear information and are optimally managed. Areas in which such coordinated work is most important are discussed in this chapter. They include the management of stone disease, the surgical approach to urinary tract obstruction, the investigation of hematuria, and the management of urinary tract malignant neoplasms.
Surgical Management of Stone Disease
The management of urinary tract stones has been irrevocably changed by the introduction of extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy (PCNL), and ureteroscopy.
Such advances have rendered open stone surgery an increasingly rare management option that is now second- or third-line treatment when indicated. Table 61-1 details the use of different treatment modalities over time since the introduction of newer techniques.1 As familiarity with available techniques and technology develops, standardized treatment strategies will undoubtedly evolve. At present, however, there remains controversy over the optimal treatment for a variety of stone patients, the specifics of which are beyond the scope of this text.
Table 61-1
Changing use of techniques for stone removal.
The changes in the application of surgical techniques for stone removal since the introduction of extracorporeal shock wave lithotripsy (ESWL) and percutaneous nephrolithotomy (PCNL).
| Changing Use of Techniques for Stone Removal | |||
| 1984 | 1990 | 1999 | |
| Location (%) | |||
| Calyceal stones | 35 | 43 | 46 |
| Pelvic stones | 42 | 20 | 13 |
| Staghorn stones | 8 | 3 | 1 |
| Ureteral stones | 15 | 34 | 40 |
| Treatment Modality (%) | |||
| ESWL | 60 | 79 | 78 |
| PCNL | 20 | 5 | 2 |
| Ureteroscopy | 11 | 15 | 20 |
| Open surgery | 9 | 1 | 0.1 |

(Data from reference 1.)
Advances in Imaging for Urinary Tract Stones
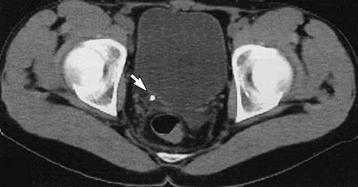
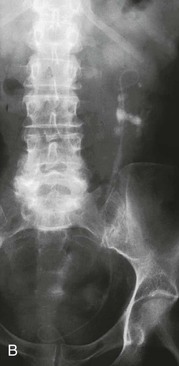
In recent years, unenhanced computed tomography (CT) scanning of the abdomen and pelvis has replaced intravenous urography (IVU) as the standard imaging modality for stone diagnosis (Fig. 61-1). CT offers increased sensitivity compared with IVU (99% vs. 70%) without the need for the intravenous administration of contrast material. An additional advantage is the ability of CT to demonstrate radiolucent stones (mainly uric acid and xanthine stones) and to detect concomitant lesions and/or alternative diagnoses. CT does require an increased radiation dose, but this is lessening with modern machines. Comparative doses are 0.42 mSv for plain radiography of the kidneys, ureters, and bladder (KUB); 2.5 mSv for IVU; and 4 mSv for non–contrast-enhanced CT.
Treatment of Urinary Tract Stones
Spontaneous stone passage can be expected in up to 80% of patients with a stone size smaller than 4 mm. Conversely, for stones with a diameter of more than 7 mm, the chance of spontaneous stone passage is very low. The location is also important; up to 70% of distal ureteral stones pass spontaneously, in contrast to only 45% of midureteral and 25% of proximal ureteral stones. Intervention is strongly recommended when there is persistent pain (for more than 72 hours) despite adequate analgesia, persistent obstruction with risk of impaired renal function (e.g., with preexisting renal impairment or in a single kidney), bilateral obstruction, or associated urinary tract sepsis.
In the absence of an acute indication for surgical management, medical expulsive therapy (tamsulosin 400 µg once daily, nifedipine 30 mg once daily), which relaxes the distal ureter and increases the likelihood of stone passage, is now commonplace in many centers. There is a growing body of evidence to support the use of medical expulsive therapy in ureteral stone disease. However, there is inconsistency in reported studies (with respect to stone size and location), and as yet outcomes of well-conducted randomized controlled trials (RCTs) are still awaited.
Another conservative treatment option is chemolysis, as several stone types are in principle amenable to dissolution by oral medications or by direct instillation of chemical solutions. However, in most patients this form of treatment is either impractical or clinically ineffective. The major exception is patients with uric acid stones, which can readily be dissolved by alkalization of the urine, usually with oral potassium citrate. (Commercially available preparations typically include potassium citrate 30% and citric acid monohydrate 5% to be diluted in water for a dose of 10 ml three times daily.)
Acute Surgical Intervention
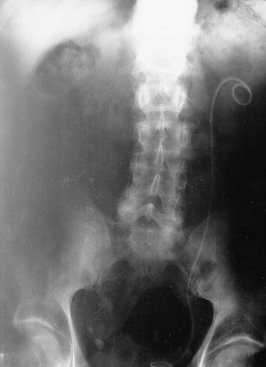
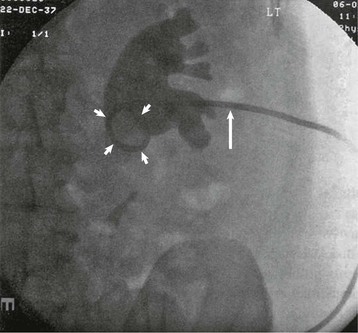
The main goal of acute surgical intervention is to relieve obstruction. If the patient is considered well enough for general anesthesia, ureteroscopic stone destruction can be attempted in most cases. Alternatively, a double-J stent (a ureteral stent with two coiled ends) can be inserted, which will relieve obstruction until definitive treatment is performed (Fig. 61-2). However, in the setting of uncontrolled urinary tract infection, percutaneous nephrostomy (PCN) is the preferred option because it can be performed with local anesthesia and is less likely than endoscopic surgery to cause septicemia (Fig. 61-3). Rarely, an open procedure to remove a stone or a grossly infected kidney may be necessary.
Elective Surgical Intervention
Extracorporeal Shock Wave Lithotripsy
During ESWL, acoustic shock wave energy is delivered to a stone under fluoroscopic or ultrasound guidance. Treatment sessions typically last about 30 minutes, during which 1500 to 2500 shock waves are delivered. Sessions are performed on an outpatient basis with the patient under analgesia or intravenous sedation and can be repeated at intervals of 10 to 14 days. Stones up to 20 mm in size can be treated effectively, and stone-free rates of 60% to 98% have been reported. However, ESWL is operator dependent, and outcome is influenced by the size, composition, and location of the stone and the type of lithotripter used. Cystine and calcium oxalate monohydrate stones are especially resistant. Targeting of the stone may be impossible in the presence of obesity and skeletal deformities (increased skin-to-stone distance), and ESWL is contraindicated in patients with aortic or renal artery aneurysm, uncontrolled urinary tract infection, or coagulation disorders and in pregnant women.
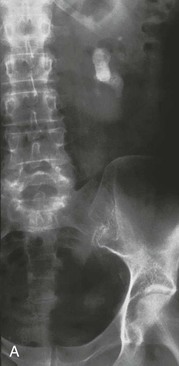
A double-J ureteral stent is sometimes placed endoscopically before ESWL treatment to prevent stone fragments from obstructing the distal ureter (Steinstrasse, literally “stone street”; Fig. 61-4). Other acute complications of ESWL include hemorrhage or hematoma, infection, and injury to adjacent organs. The risk for the later development of hypertension or renal impairment after ESWL remains controversial.
Extracorporeal shock wave lithotripsy is the first-line treatment for more than 75% of stone patients. Table 61-2 shows circumstances in which ESWL is less effective and PCNL becomes the preferred approach or a combination of the two modalities is used. For lower pole stones in particular, ESWL may not provide optimal clearance because of problems with the drainage of residual fragments. An RCT has shown that for lower pole stones larger than 10 mm, PCNL has much better clearance rates than ESWL (92% vs. 23%).2
Table 61-2
Indications for percutaneous nephrolithotomy.
ESWL is the first choice for stone intervention, except in those circumstances that may favor PCNL. ESWL, Extracorporeal shock wave lithotripsy; PCNL, percutaneous nephrolithotomy; PUJ, pelviureteral junction.
| Indications for Percutaneous Nephrolithotomy | ||
| Composition* | Struvite stones | Complete removal necessary to eliminate infection and minimize stone recurrence |
| Calcium oxalate monohydrate stones | Difficult to pulverize by ESWL | |
| Cystine stones | Difficult to pulverize by ESWL | |
| Stone position | Lower pole stones | Fragments less easily evacuated from dependent lower pole calyces, especially if collecting system dilated |
| Anatomic abnormalities | PUJ obstruction Calyceal diverticula | Prevent passage of fragments after ESWL |
| Patient characteristics | Morbid obesity Ureteral obstruction | Stone cannot be placed in focal point of ESWL machine |
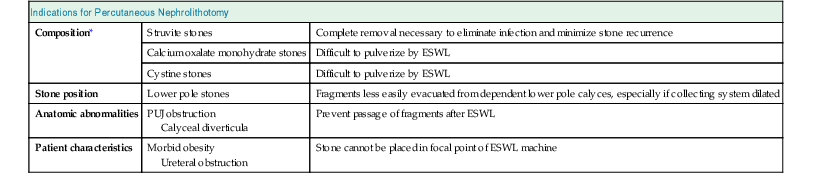
* Stone composition can be defined with certainty only by direct stone analysis, but advances in imaging may ultimately provide a means to accurately assess stone composition in situ before treatment, thus allowing the urologist to select the treatment most likely to be successful.
Percutaneous Nephrolithotomy
During PCNL, a tract is created between the skin and the collecting system of the kidney and used as a working channel to remove stones. Preoperatively, IVU or CT, sometimes with three-dimensional reconstruction, is used to accurately localize calculi and neighboring organs (e.g., spleen, liver, large bowel, pleura, or lungs) and to plan access. The most frequently used access site is the dorsal calyx of the lower pole, and stone fragmentation is undertaken by ultrasound, pneumatic, or laser devices. The PCNL technique is modified for special circumstances, usually by altering the site of puncture (e.g., directly into a calyceal diverticulum) or, if there are ureteral stones, by using a higher-placed puncture to permit antegrade ureteroscopy. The percutaneous puncture may be facilitated by the preliminary placement of a retrograde ureteral catheter to dilate and to opacify the collecting system, which is then punctured under fluoroscopy. After completion of PCNL, a self-retaining balloon nephrostomy tube is used to tamponade the tract and to provide further access if needed. Hemorrhage can complicate PCNL (from renal or, rarely, intercostal arteries) and can usually be treated conservatively or by selective angiographic embolization. Other complications include sepsis; fluid overload (similar to transurethral resection syndrome); injury to spleen, pleura, or colon; and extravasation. PCNL usually results in minimal parenchymal injury, averaging only 0.15% of the total renal cortex.3
Indications for PCNL are shown in Table 61-2. These continue to evolve and are being challenged by developments in ureteroscopic techniques, which are allowing more upper ureteral and renal pelvic stones to be dealt with by a retrograde approach.
Open Stone Surgery
Although it is performed increasingly rarely, open surgery still has a place in the treatment of stone disease. It is reported that approximately 2% of stone patients are treated with open surgery, mainly when anatomic factors preclude the use of minimally invasive methods or when these techniques have failed. Other indications include complex stone burden and the presence of intrarenal anatomic abnormalities (e.g., pelviureteral junction obstruction). During surgery the renal pelvis as well as the parenchyma can be opened along avascular planes, and clamping of the renal vessels and hypothermia of the kidney may be needed. In selected patients a laparoscopic approach can be used for the treatment of stone disease.
Ureteroscopy
Recent advances in the design of endoscopes for ureteronephroscopy have rendered the entire urinary tract accessible to endoscopic examination and manipulation. Ureteroscopes may be semirigid or flexible, the latter allowing access to the renal pelvis and calyces. Stone fragmentation is achieved ideally by laser but also by ultrasound or pneumatic devices (lithoclast). Laser use is equally effective for all types of stones and has the additional advantages of a flexible fiber (allowing intrarenal stone fragmentation), low tissue penetration, and minimal stone displacement during use. Success rates for laser fragmentation of ureteral stones are high, in the region of 80%. Figure 61-5 highlights the increasing use of ureteroscopy in stone disease and also the increasing proportion of procedures in which a laser rather than ultrasound or a lithoclast is used to achieve stone fragmentation.
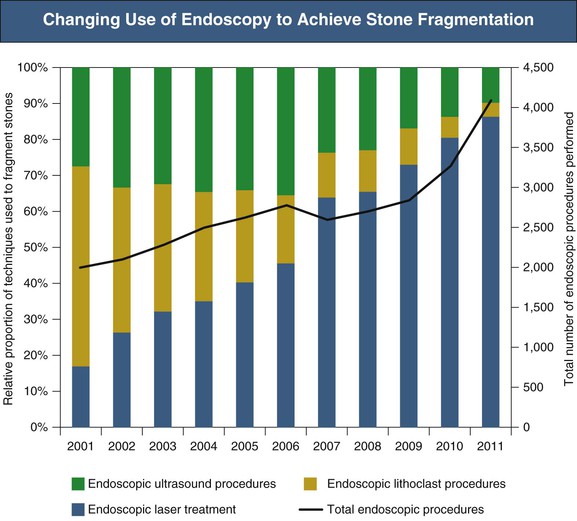
In the case of lower pole renal stones, although flexible ureteroscopes can access the lower pole to facilitate treatment, the stone-free rate in comparative studies is similar to that of ESWL.4,5 Furthermore, patient preference is often ESWL in the first instance. As a result, it is common for patients to undergo ESWL before flexible ureteroscopy and lasertripsy. In the event of failed stone clearance after ureteroscopy, PCNL is commonly used next and is likely to remain an essential treatment for lower pole stones. Complications of ureteroscopy, particularly with use of graspers and baskets, include ureteral avulsion, perforation, extravasation, mucosal damage, hematuria, infection, and stricture. Advances in laser technology now enable stones to be reduced to dust-like particles, reducing the need for graspers and baskets and hence reducing complications.
Management of Staghorn Calculus
A staghorn calculus should usually be managed by intervention because reports of conservative therapy show a high rate of eventual nephrectomy (up to 50%) and an increase in associated morbidity (mainly renal failure) and mortality (up to 28%). Surgical options are ESWL monotherapy, complete endoscopic stone removal, and a combined approach with use of PCNL for debulking followed by ESWL. The advantage of the combined approach is the reduced need for additional renal access and secondary endourologic procedures. The choice of treatment depends on many factors, including the age and renal function of the patient. ESWL will not usually render the patient entirely stone free (the ideal goal of treatment), especially in the treatment of large staghorn stones; nevertheless, it can be considered successful when less than 40% of a staghorn calculus persisting as fragments is achieved. Rarely, open surgical removal of the stone may be indicated.
Stones in Transplanted Kidneys
The management of stone disease in a transplanted kidney is challenging because of the solitary kidney, the anatomic location within the pelvis, and the difficulty with retrograde access to the ureter and kidney. Early active intervention is indicated; prophylactic stenting, ureteroscopy, and PCNL are preferred to ESWL because stone targeting may not be possible. Open surgery may be needed in selected cases.
Management of Urinary Tract Obstruction
The causes of upper tract obstruction are listed in Chapter 60, Table 60-1, and a summary of the management of obstruction is given in Chapter 60, including Figure 60-7. Upper tract obstruction caused by malignant disease can be a result of direct tumor invasion or external compression by metastatic lymph node involvement or, rarely, true metastasis to the ureter. Some 70% of tumors causing ureteral obstruction are genitourinary (cervical, bladder, prostate) in origin; breast and gastrointestinal carcinomas and lymphoma constitute the majority of the remainder.6 The presentation of a patient with obstruction may vary significantly, and hydronephrosis may develop progressively and insidiously and remain unrecognized until the patient develops anuria and uremia. Upper tract obstruction from malignant disease rarely presents with classic acute ureteral colic, which is typically seen with a benign cause such as a stone. Bladder outflow obstruction may be acute and dramatic, or chronic with few or no symptoms. To aid in understanding of the immediate needs and longer-term prognosis of these patients, the acute as well as the definitive management of the most common urologic diseases associated with obstruction and renal impairment are outlined.
Acute Management of Urinary Tract Obstruction
Relief of obstruction is crucial to reverse renal impairment and to preserve remaining renal function. In cases of bladder outflow obstruction, a urethral or suprapubic catheter is indicated; whereas in upper tract obstruction, a double-J ureteral stent is preferable, when possible. The most straightforward approach is endoscopic retrograde placement under fluoroscopy, with PCN reserved for patients in whom the procedure fails. Bilateral stents should be placed if it is technically possible. However, tumor infiltration can distort trigonal anatomy, making identification of ureteral orifices for double-J stent insertion impossible at the time of cystoscopy. Furthermore, it has been suggested that stents fail to relieve obstruction in 40% to 50% of cases of external ureteral compression. Thus, these patients need to be closely monitored to ensure resolution of the obstruction. A new type of metallic, self-expanding stent, used alone or in conjunction with double-J stents, has had good results in maintaining ureteral patency and avoiding PCN in malignant ureteral obstruction.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree