Chapter 31 URETHRAL INJECTABLES IN THE MANAGEMENT OF STRESS URINARY INCONTINENCE
HISTORICAL BACKGROUND
Urethral bulking agents have been used for many years to treat intrinsic sphincter deficiency. They are minimally invasive alternatives to operative procedures such as anterior repairs, suspensions, and urethral slings for the management of stress urinary incontinence (SUI). Urethral injection, which can be delivered under local anesthesia as an outpatient procedure, is relatively safe and has few complications. It is an effective treatment for SUI, with complete patient satisfaction comparable to surgery. Urethral injection is cost-effective with less operating time and a shorter hospital stay compared with more invasive surgery.1,2
The concept of urethral injectables to increase urethral resistance has been known for 70 years. Murless,3 in 1938, was the first to report his experience with injection of the sclerosing agent sodium morrhuate into the anterior vaginal wall in 20 patients to induce an inflammatory reaction that compressed the urethra with sclerosis, leading to destruction of the urethral musculature and decreased the compliance of the urethral wall. Quackels,4 in 1955, injected paraffin wax perineally in two incontinent patients after prostatectomy. Dondren is another sclerosing agent that has been used for urethral injection with some success.5
The injection of sclerosing materials such as sodium morrhuate, paraffin, or Dondren causes unacceptable complications, including sloughing of the urethra, urethral stenosis, and pulmonary embolism.5 Polytetrafluoroethylene (Teflon) was proposed as the first bulking agent by Lopez and colleagues8 in 1964, and it became popular in the 1970s.6,7 Since then, several bulking agents have been developed, and new ones are being studied. Nonautologous substances, such as collagen and hyaluronic acid, and autologous substances, such as fat, chondrocytes, and muscle, have been employed clinically or are still under investigation. This chapter reviews the safety and efficacy of available injectable agents for the treatment of female SUI.
MECHANISM OF ACTION OF BULKING AGENTS
The mechanism of continence in urethral injection therapy is uncertain and controversial. Many factors enable normal continence in females, including contraction of the sphincter muscles, musculofascial support of the bladder neck, and a urethra seal mechanism.8 The functional urethral seal mechanism is probably a major contributor to continence because of its ability to increase urethral resistance and urethral opening pressure during coughing and straining. Common causes for loss of the urethral seal mechanism are scarring from previous operations, birth trauma, estrogen deprivation, or pelvic radiotherapy.9 Submucosal urethral injection of bulking materials augments bladder neck length and urethral mucosa coaptation and improves the closure mechanism of the urethral sphincter in response to increased intra-abdominal pressure.10 Some investigators suggest an obstructive mechanism for the action of urethral injectables, as witnessed sometimes by a decrease in the maximum flow rate and heightened voiding maximum detrusor pressure after injection.11,12 Several laboratories have reported elevation of Valsalva leak point pressure (VLPP) as a result of an increase in functional urethral length.13–15 Others have found that bulking materials improve the ratio of urethral resistance to abdominal pressure by raising the VLPP but not the detrusor leak point pressure or voiding pressure.16,17 Monga and Stanton18 postulated that prevention of bladder neck opening during stress is the mechanism of action of collagen injection, not obstruction.
Cephalad elongation of the urethra caused by bulking agent injection at the bladder neck or proximal urethra probably accounts for increased abdominal pressure transmission in the first quarter of the urethra.18,19 Placement of the injectable material more distally does not increase the functional length of the urethra or prevent bladder neck opening during episodes of stress. It is suggested that the bulking materials should be placed just distal to the urethral–vesical junction and that the position of the injectable is more important than its quantity for a good bulking effect.19,20
INDICATIONS AND CONTRAINDICATIONS
Injectable agents are classically indicated in patients with SUI. Several studies have shown good efficacy for bulking agents, even in the presence of bladder neck mobility. There is a trend to admit some degree of intrinsic sphincter deficiency (ISD) in all cases of SUI. Bulking agent indications then become much broader, and physicians are influenced by other parameters, such as patient preferences, cost, the need for concomitant procedures (e.g., for prolapse), and product availability.22–25
An overactive bladder should be treated before undertaking urethral injection. Many physicians believe that untreated bladder instability and low compliance are contraindications to urethral injection; others think that urethral injection can be used to treat the incompetent outlet of an overactive bladder without adverse effects on clinical outcome.26
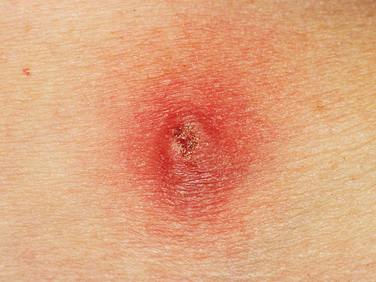
Contraindications to urethral injections are untreated urinary tract infection and hypersensitivity to the injectable materials (Fig. 31-1). Extensive scarring of tissue from irradiation or previous surgery or trauma may decrease the retention of injectable material in tissue, leading to a poor outcome.
INJECTION TECHNIQUE
The goal of injection therapy in SUI patients is restoration of the urethral submucosal “cushion” to improve urethral closure pressure during stress episodes without compromising voiding detrusor pressure. The advantages of injection therapy are its minimal invasiveness and technical simplicity, allowing delivery in an outpatient facility under local anesthesia. Urine culture for all patients should be negative, and if collagen is chosen, a skin test to rule out hypersensitivity to collagen must be performed at least 4 weeks before the treatment (see Fig. 31-1). Perioperative antibiotics (e.g., one dose of extended-release quinolone) are usually recommended. After having emptied her bladder, the patient is installed in the lithotomy position, prepared with antiseptic cleaning solution at the level of the external genitalia and urethral meatus, and then draped as usual.
Three injection methods have been proposed: periurethral, transurethral or intraurethral, and antegrade.27 Only the transurethral technique is still in use.
Periurethral Technique
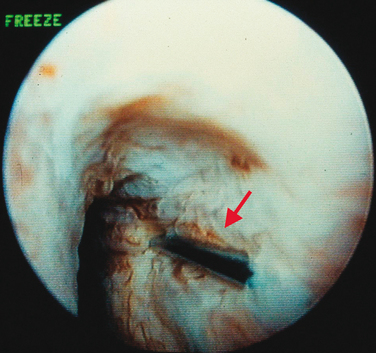
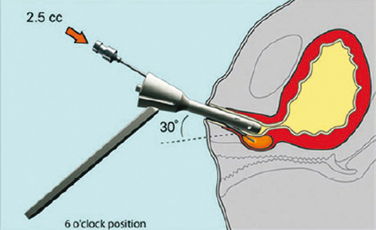
Injections are usually administered with instillation of transurethral topical anesthetic (i.e., 2% lidocaine jelly) and 3 to 4 mL of 1% lidocaine injected in the periurethral tissues at the 3- and 9-o’clock positions. A 20-gauge needle connected to the syringe of bulking agent is successively inserted slowly at the 3-, 6-, and 9-o’clock positions and advanced into the submucosal tissues (i.e., layer just under the urothelium), approximately 0.5 cm distal to the bladder neck, to raise a urethral bleb. Needle position is controlled by urethroscopy under a 0-degree lens. At each position, the bulking agent should be injected slowly to avoid rupturing the expanding urothelium. The injection is continued until the mucosa appears pale and the lumen is about 30% occluded.24 To ensure success, visualization of complete coaptation (i.e., bilateral kissing lobes) of the urethral mucosa at the end of the procedure is recommended (Fig. 31-2). For some physicians, this technique can also be done under ultrasonic guidance.28 In some cases, positioning of the needle can be difficult, leading to repeated mucosal perforations (Fig. 31-3).
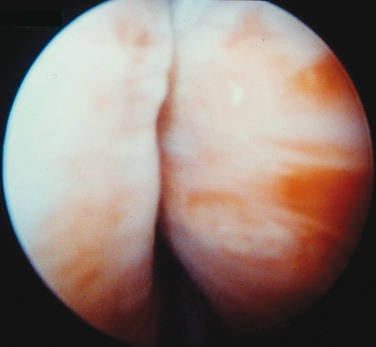
Figure 31-2 Coaptation of the urethra after injection of bulking agents at the 3- and 9-o’clock positions.
Transurethral or Intraurethral Technique
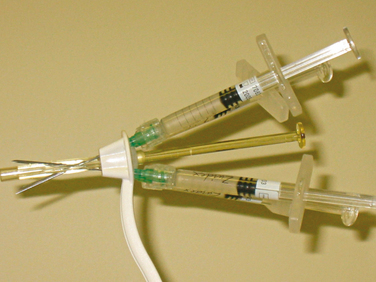
Two transurethral techniques are available: the cystoscopically guided technique and the blind technique. In the first one, needle insertion into the submucosal tissue is achieved under cystoscopic vision with different injection devices. It requires a cystoscope or a urethrocystoscope with an injection needle (needle size depends on the viscosity of the injectable material) and usually a 0- or 12-degree lens.29 The needle should be inserted into the submucosal space approximately 0.5 to 1.5 cm distal to the bladder neck at the 3-, 6-, and 9-o’clock positions. The bulking agent is injected slowly until it raises a urethral bleb. Because of the high viscosity of some injectable agents, such as Teflon or silicone microparticles, an injection gun may be necessary (Fig. 31-4). After injection, the physician must avoid advancing the cystoscope beyond the injection site to prevent compression and extrusion of the injectable material.
Faerber and associates30 compared the intraurethral and periurethral routes and demonstrated significant differences in cure (46% versus 33%) and improvement (50% versus 67%) rates in favor of the intraurethral route. However, Schulz and colleagues31 compared periurethral and intraurethral injection randomly in 40 women and concluded that both routes of injection were equally effective. In Faerber’s report, it was evident that the intra-urethral route required much less injectable material (4.7% versus 10.1 mL) for better results. The average number of sessions (1.3) was identical with both approaches. These findings explain why most centers now use only the intra-urethral approach.
The blind transurethral technique uses injection devices that do not require cystoscopic guidance. Henalla and coworkers32 were the first to apply Macroplastique implantation system to improve and simplify the injection technique (see Fig. 31-4). With this device, the success rate was 74.3%, and the rate of acceptability by surgeons was 95%. The implantation device was developed to control accurate placement of the bulking agent at predefined sites and depth in the female urethra without the need for cystoscopic guidance.33 The device is advanced into the urethra until urinary drainage is established and then withdrawn 1 cm. The injection needle is inserted into the first deployment site, angling the device in the direction of the injection site to ensure penetration of the mucosa.34 The device enables consistent bolus placement at a predetermined depth and site. Usually, two to three punctures (at the 4-, 8-, and 12-o’clock positions) are needed for material delivery. If injection treatment fails, transvaginal or transurethral ultrasound can be performed to investigate correct Macroplastique placement. A different version of the device has been introduced by Q-Med AB (Uppsala, Sweden) (Fig. 31-5). The Implacer device has been developed for administration of dextranomer/hyaluronic acid (Dx/HA; Zuidex) without the need for cystoscopic guidance. The Implacer uses four syringes (each containing 0.7 mL of Dx/HA) and 23-guage needles. The device unfolds and fixates the urethral wall to ensure symmetric placement of the injectable agent at four evenly spaced locations around the urethra.
POSTOPERATIVE CARE
Patients are usually discharged to their homes after satisfactory voiding. If urinary retention occurs, a small-caliber catheter (14 Fr or smaller) is inserted to empty the bladder. In rare cases of persistent retention, intermittent catheterization with a small-caliber catheter is required until normal micturition resumes.24 Mechanical pressure to the perineum (e.g., hard seat covers, hard stools, intravaginal sexual intercourse) should be avoided for 2 weeks. If a second injection is necessary, it should be performed after a few weeks to allow healing of the previous implant.28
ASSESSMENT OF CLINICAL OUTCOMES
No validated, reproducible, well-accepted instruments have existed for the assessment of outcomes of treatment of urinary incontinence.35 There is no standard definition of success in studies of anti-incontinence procedures, making it difficult to objectively compare results. Assessment of cure depends on subjective or objective measurements, which are not correlated. In most reported studies, cure is defined as the patient being dry by the end of the follow-up period. Improvement is defined as rare or minimal leakage and patient satisfaction with the result of the injection.36
Initial patient assessment should include a symptom evaluation, patient satisfaction score, quality-of-life questionnaire, leakage gravity index (e.g., pad test, visual scale), physical examination, and urodynamic measurement. Follow-up should monitor the same parameters plus the length of time since the last injection, number of injections performed, volume per injection, and cure criteria (e.g., physical examination, pad usage, pad-weighing tests, urodynamics).37
INJECTABLE MATERIALS
Ideal bulking agents should be biocompatible, biodegradable, nonmigratory (bulking > 80 μm), nonerosive, noncarcinogenic, nonimmunogenic, permanently bulking, and easily injected.25 Unfortunately, none of the available bulking agents entirely meets these requirements.
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE; Teflon, Polytef) was described as a bulking agent by Lopez and colleagues in 1964,8 and it was popularized by Berg6 and Politano7 in 1970. It is a paste consisting of colloidal PTFE micropolymeric, various-sized particles (up to 300 μm, with most being < 50 μm).8 It is an inert, stable material with a high molecular weight and high viscosity, and it is nonallergenic.
Teflon has several disadvantages that limit its acceptability and prevent it from being approved by the U.S. Food and Drug Administration (FDA) for periurethral injection in the United States. It is difficult to inject because of its density, requiring very high pressure through a large-bore needle. Because of the small size of the particles, PTFE (90% < 40 μm in diameter) can be phagocytosed, resulting in distant migration to the brain, lungs, and lymph nodes.38,39 Among other major inconveniences, PTFE is not biodegradable, and it carries a risk of granuloma formation at the injection site and at some sites of distant migration.40 PTFE produces an inflammatory reaction that may lead to urethral fibrosis, periurethral abscess, and urethral diverticulum.41 The carcinogenic potential of PTFE injection has been suggested but not proved and never reported clinically.42
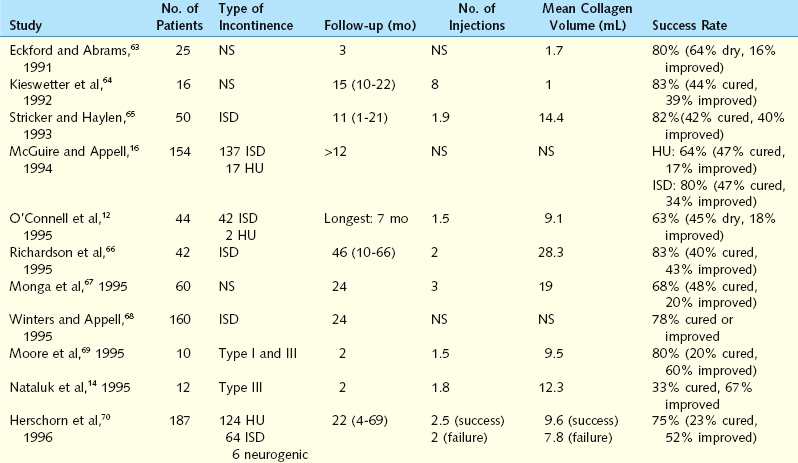
Politano and coworkers43 first used Teflon for incontinence in men after prostatectomy and later for stress incontinence in women. Their short-term results were promising, with cure and improvement rates of 57% to 85%. However, long-term data have ranged from 18% to 76%.44–46 The reasons for failure of Teflon injection include the high pressure needed for injection, leading to tissue extrusion, absorption, and migration of Teflon particles, and the inflammatory reactions affecting urethral function.37 Failures in this series were associated with prior incontinence operations and bladder instability. To minimize the risk of migration, Herschorn and Glazer47 evaluated the injection of low-volume Teflon, with a success rate of 71.7% (Table 31-1).
Glutaraldehyde Cross-Linked Collagen
Bovine glutaraldehyde cross-linked collagen (Contigen) is the most widely studied bulking agent. It is a well-established injectable material that gained FDA approval in 1993.24 Periurethral injection of glutaraldehyde cross-linked collagen was first reported by Shortliffe and associates in 1989.55 Contigen, a highly purified suspension of bovine collagen in normal saline, contains more than 95% type I collagen and less than 5% type III collagen cross-linked with glutaraldehyde. This cross-linking makes collagen more stable and durable, hindering its breakdown by fibroblast-secreted collagenase and enhancing its invasion by fibroblasts and blood vessels with deposition of host collagen, promoting long-term efficacy of the implant.56 The decreased antigenicity of this mixed collagen is obtained by hydrolysis of the antigenic parts of the molecules, the amino-terminal and carboxyl-terminal segments. Cross-linking also reduces hypersensitivity.
Collagen injection is an easy procedure. It can be delivered periurethrally or transurethrally through a small needle (22 gauge) under local anesthesia. Each syringe contains approximately 2.5 mL of collagen, and some patients may need repeated injections (e.g., two to five injections). If the collagen is placed too deeply, it will be quickly reabsorbed. However, the position and volume of injected collagen are not predictive of clinical outcome, as determined by a magnetic resonance study.57,58 The advantages of collagen injection include its durable efficacy and safety, with no proven risk of granuloma formation or migration.59 Considering its price, cost-effectiveness is a concern. Berman and Kreder60 reported that sling cystourethropexy is more cost-effective than collagen injection in women with type III incontinence. The collagen injection is allergenic in up to 5% of patients, requiring skin tests to rule out hypersensitivity 1 month before injection.61 There also is some concern regarding the potential for disease transmission from bovine products.34
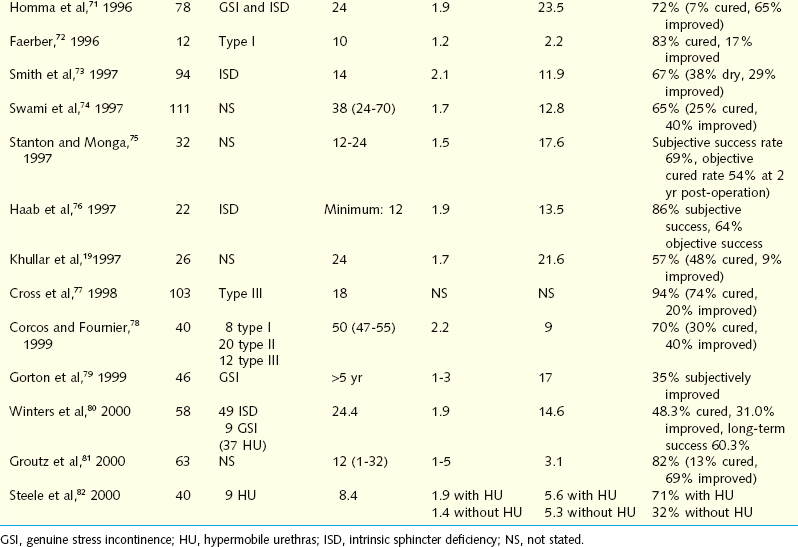
Early results have disclosed subjective cure and improvement rates up to 95% and objective cure rates of 61% to 91%.36 Herschorn and colleagues62 reported an early experience with intra-urethral injection of collagen in 31 women, with a mean follow-up of 6 months. The combined cure and improvement rate was 90.3%. Longer-term results of cure and improvement rates vary from 57% to 94% (Table 31-2). In a North American multicenter study, 148 women underwent collagen injection for ISD, with an overall success rate of 78% (45% cured, 33% improved) at 2 years of follow-up.63 Monga and coworkers67 achieved a success rate of 86% at 3 months, 77% at 12 months, and 68% at 24 months after urethral injection of collagen in 60 women with SUI. Swami and associates74 treated 111 patients with periurethral collagen injection for a success rate of 85% at 6 months and 65% at 3 years after injection. There was no dif-ference in maximum urethral closure pressure before and after collagen injection and no predictive factors of success. Some physicians have reported that patients who underwent prior antiincontinence surgery may have better results than those without previous surgery.63,65 These findings may be explained by periurethral tissue support and limited mobility by scarring.63,65 Others have observed no correlation between the degree of urinary incontinence preoperatively and the success rate.70 Some investigators have obtained higher percentages of injection failure in patients with preoperative detrusor instability.70,73 The use of collagen in cases of urethral hypermobility has produced results comparable to those without hypermobility.16,67,69,70,72 Steele and colleagues82 reported a higher success rate for collagen injection in patients with hypermobility than in those without hypermobility.
Long-term studies have demonstrated continuing success, with a cure rate of 25% to 45% and an improvement rate between 25% and 50%. Corcos and Fournier78 reported the 4-year follow-up results for 40 women who underwent collagen injection. The cure rate was 30%, and the improvement rate was 40%. One third of these patients required a top-up injection 18 to 24 months after the initial treatment. Gorton and coworkers79 published their long-term results of collagen injection in 46 women with ISD, showing an overall success rate of 35%.
The morbidity associated with collagen injection is minimal and self-limited. The most common complication is transient urinary retention, urinary tract infection, and transient hematuria. In 337 collagen injection cases, Stothers and associates83 documented a complication rate of 20%, including de novo urinary urgency in 12.6% of patients. In many patients, the symptoms were irreversible (21% did not respond to anticholinergics) and included hematuria (5%) and urinary retention (1.9%). Delayed reaction at the skin test site occurred in 0.9% (3) of patients and was associated with arthralgia in two cases. Others identified urinary retention (8%,) urinary tract infection (4%), hematuria (2%), and urinary urgency in less than 1%.84 Rare complications after collagen injection included sterile abscess at the injection site.85,86 Pulmonary embolism, urethral prolapse, and osteitis pubis have been encountered after urethral collagen injection.86–88
Autologous Fat
Autologous fat was first used in plastic surgery to augment soft tissue defects. Periurethral fat injection was introduced in 1989 by Gonzales and colleagues89,90 to treat ISD. Fat was harvested from lower abdominal subcutaneous tissue by liposuction using a trocar or aspiration syringe under local or general anesthesia. Between 15 and 20 mL of fat was mixed with Ringer’s solution or insulin before periurethral or transurethral injection. The advantage of autologous fat is that it is readily available, biocompatible, and inexpensive. Its disadvantages are fat resorption and replacement by fibrous tissues, which necessitate repeated injections. Horl and coworkers91 employed magnetic resonance imaging to demonstrate a 55% volume loss at 6 months after fat injection but no further volume decline at 9 and 12 months of follow-up. Approximately 50% to 90% of transferred adipose grafts do not survive.92 Graft survival depends on minimal handling, low suction pressure during liposuction, and large-bore needles for reinjection to minimize injury.93
Periurethral fat injection has a reported success rate that is lower than with other injectables (Table 31-3). Palma and associates97
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree