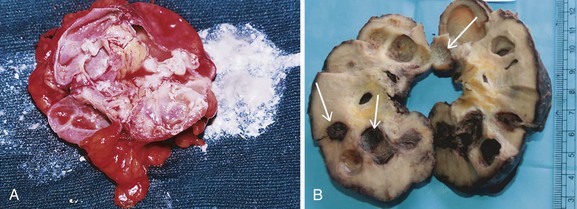
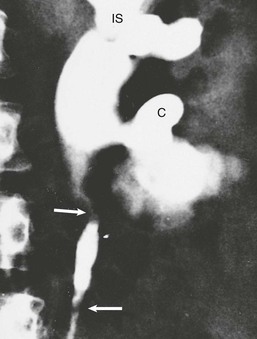
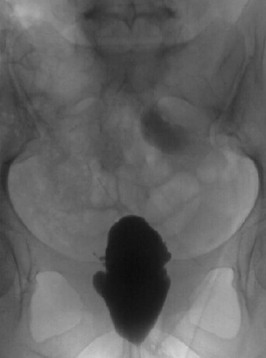
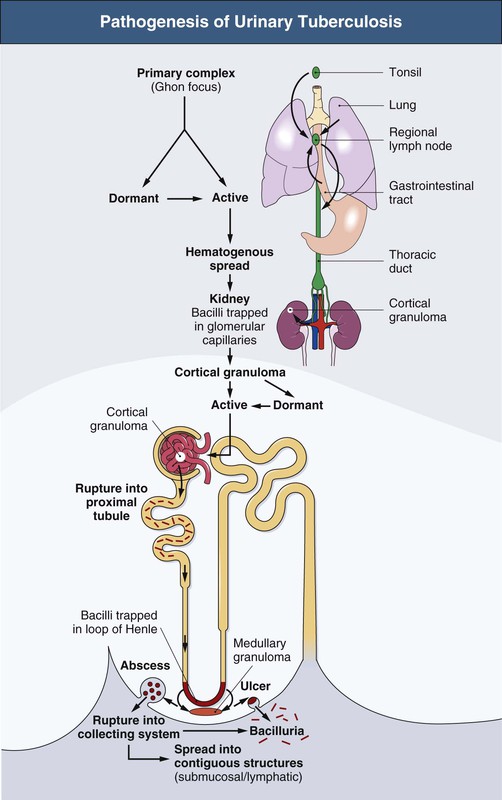
R. Kasi Visweswaran, Suresh Bhat Tuberculosis (TB), caused mostly by Mycobacterium tuberculosis, is a major global health problem. According to a recent report by the World Health Organization (WHO), there were almost 9 million new cases in 2011 and 1.4 million deaths attributed to TB.1 From 1999 to 2020, there will be an estimated 1 billion new cases of TB if control measures are not improved. In developed countries, TB usually affects older individuals and immigrants from countries with high prevalence. The incidence of TB is 100 times greater in patients with human immunodeficiency virus (HIV), and TB is the most common opportunistic infection in HIV-infected patients.2 TB also is more common in patients with chronic kidney disease (CKD), especially when associated with anatomic abnormalities or immunosuppression. In some endemic areas, TB has been reported to occur in up to 9% of patients receiving hemodialysis, 9% of renal allograft recipients, and 12% of children with nephrotic syndrome.3 Genitourinary TB occurs in about 5% of active TB cases in the non–HIV-infected population.4 It is almost always secondary to a symptomatic or asymptomatic primary lesion in the lung. Renal involvement may also occur as a complication of miliary (septicemic) TB. In recent years, the incidence of multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB has increased. According to WHO estimates, there were 630,000 cases of MDR TB in 2001.1 This scenario has compromised programs to eradicate TB. The tubercle bacillus is a nonmotile, nonsporing, strictly aerobic straight or slightly curved rod-like bacillus that is weakly gram positive and acid and alcohol fast. Mycobacteria have a lipid shell (“lipid barrier”) containing mycolic acid that resists proteolysis and uptake into phagolysosomes; muramyl dipeptide, which stimulates a T cell response that elicits the characteristic granuloma; and cell wall glycolipids that inhibit macrophage function. This surrounding coat of inert lipids and surface proteins allows mycobacteria to survive inside phagocytes, where they may remain dormant for years.5 Whereas most TB including genitourinary TB is caused by M. tuberculosis,5 other mycobacteria may rarely cause clinical disease, especially in immunocompromised hosts. These include M. avium-intracellulare, M. kansasii, M. bovis, M. fortuitum, and M. szulgai. The clinical and pathologic manifestations of TB depend on the virulence of the organism and the effectiveness of the host response. The host response may lead to complete containment of infection or result in an illness of varying severity. Strain differences may also determine whether an infected person develops primary TB, reactivation TB, or a chronic asymptomatic infection. A low serum level of 25-hydroxyvitamin D may also compromise cell-mediated immunity and increase the risk for activation of latent TB. When an infected droplet with the size of 1 to 5 µm is deposited in the respiratory tract, tonsillar fossa, or gastrointestinal tract, a primary focus develops with the formation of a nonspecific, asymptomatic granuloma. The organisms from the primary focus drain to the regional lymph gland, causing its enlargement, resulting in the primary complex. This is often asymptomatic and self-limited. Bacilli from the regional lymph node may also enter through the thoracic duct into the blood, resulting in silent dissemination to various sites, including the cortex of the kidneys (Fig. 54-1). Here the bacilli elicit an inflammatory response, resulting in granuloma formation that may heal and form a scar, remain dormant for many years, or rupture into the proximal tubule of the nephron. The bacilli in the nephron are trapped at the level of the loop of Henle, where they multiply. The relatively poor blood flow, hypertonicity, and high ammonia concentration in the renal medulla impair the immune responses and favor the formation of medullary granulomas. These granulomas (tuberculomas), which contain macrophages, may undergo coagulative necrosis, forming cheese-like caseous material (Fig. 54-2), and occasionally rupture into the calyx.6 The renal medulla is the most common site of involvement of clinical renal TB and is usually unilateral.7 When this caseous focus ruptures into the collecting system, cavities and ulcers are formed, and involvement of renal papillae may lead to sloughing and papillary necrosis. Healing in the kidney occurs by fibrosis and scarring, resulting in strictures and obstruction. Calcification commences intracellularly by the accumulation of phosphate ions from the disintegration of nucleoproteins and calcium ions from cell membrane damage. These lesions may harbor live mycobacteria, and such dystrophic lesions should be considered active disease and not a sign of healing. Dystrophic calcification of damaged structures may result in a nonfunctioning kidney called “putty” or “cement” kidney. Spread of TB to contiguous structures may occur; ureteritis is common and may result in strictures and obstructive uropathy (Fig. 54-3). The bladder may develop hyperemia near the ureteral orifice, followed by superficial ulcers and granulomatous changes involving all layers (pancystitis). Healing by fibrosis at the ureteral orifice results in a refluxing “golf-hole” ureter. Extensive fibrosis of the bladder wall results in a thick, small-capacity bladder called “thimble” bladder (Fig. 54-4). Bladder infection may also rarely result from instillation of bacille Calmette-Guérin (BCG) in the bladder as part of treatment of superficial bladder carcinomas. Involvement of the genital tract is also common. As many as 70% to 80% of men with TB of the urinary tract have epididymitis, prostatitis, seminal vesiculitis, orchitis, or cold abscesses. In women, genital tract involvement is less common; but if present, it usually presents as salpingitis, often diagnosed during investigation for infertility. Transplanted kidneys may transmit TB to their recipients.8 Urinary tract TB may be asymptomatic or may mimic other disorders. Patients may present with constitutional symptoms or symptoms related to the lower urinary tract, abdomen, or genitalia (Table 54-1). A high index of suspicion enables early diagnosis. Most patients are 20 to 40 years of age, with a male-female ratio of 2 : 1. Because active genitourinary TB presents 5 to 15 years after primary infection, it is relatively rare in children. Risk factors for TB include close contact with sputum smear–positive individuals, vagrancy, social deprivation, neglect, immunosuppression, HIV infection or acquired immunodeficiency syndrome (AIDS), diabetes mellitus, CKD, vitamin D deficiency, and other debilitating illness. Table 54-1 Clinical features of urinary tuberculosis. Almost 25% of patients have no clinical or laboratory evidence of abnormality, and the diagnosis of urinary TB is made on investigation for other diseases, during surgery, or at autopsy. Another 25% have asymptomatic urinary abnormalities, usually persistent asymptomatic pyuria or hematuria. In patients with persistent pyuria, conventional urine cultures do not yield growth, and the urine is usually acidic; thus the term acid-sterile pyuria. Of the patients who are symptomatic, lower urinary tract symptoms, such as frequency, urgency, dysuria, nocturia, frank pyuria, or hematuria, occur in more than 75% of TB patients. Increased urinary frequency is an early symptom and results from inflammation of the bladder. The defect in the urinary concentrating mechanism explains the nocturia. Recurrent bouts of painless macroscopic (gross) hematuria should alert the clinician to the possible diagnosis of urinary TB, although glomerular diseases such as IgA nephropathy should also be considered. Macroscopic hematuria in urinary TB is a result of bleeding from the ulcerating lesions, inflammation of the urothelium, or rupture of a blood vessel in the vicinity of a cavity. Colicky pain may occur as a presenting manifestation of urinary TB when it is associated with stone, clot, sloughed papilla, or other causes of acute obstruction. In advanced disease, frequency and urgency related to reduced bladder capacity (thimble bladder) occur. Incomplete emptying, increased susceptibility to infection, and secondary vesicoureteral reflux (VUR) may also occur. In chronic ureteral obstruction, an enlarged kidney, infection, or perinephric collection leads to a dull-aching loin pain. Severe suprapubic pain with backache and dysuria suggests acute tuberculous cystitis. Patients with TB cystitis may show worsening of frequency and urgency after antituberculous treatment because of fibrosis and bladder wall contraction and is a part of healing process. This should not be mistaken for a nonresponse to therapy. Episodes of pyuria, which may suggest secondary bacterial infection or drainage of a caseous focus into the collecting system, may also be a manifestation of renal TB. Persistence of pyuria after appropriate therapy should lead to an evaluation for urinary TB. Longstanding renal TB may result in mild tubular proteinuria (<1 g/24 h) in up to 50% of patients. About 15% have proteinuria of more than 1 g/24 h, and nephrotic syndrome caused by amyloidosis may develop in some patients. Rare cases of mesangial proliferative glomerulonephritis have also been reported. Anemia is seen in less than 20% of patients with nonmiliary disease, but the frequency is higher in those with CKD.9 A few patients have nephrogenic diabetes insipidus. Renal tubular acidosis may occur. Hyporeninemic hypoaldosteronism may result from the tubulointerstitial injury secondary to obstructive uropathy.10 Renal function is usually normal, but CKD may develop if both kidneys are extensively damaged. Some patients with urinary TB present with reduced glomerular filtration rate (GFR), pyuria, microscopic hematuria, and proteinuria, but the urine cultures for mycobacteria are repeatedly negative. These patients respond favorably to antituberculous chemotherapy combined with corticosteroids. The kidneys are of normal size and show diffuse interstitial nephritis, with caseating granulomas containing the bacilli in 75% of the biopsy samples.11 Hypertension is unusual in renal TB, but intimal proliferation of vessels near inflamed areas may lead to segmental ischemia and renin release.12 In patients with a nonfunctioning kidney, nephrectomy may help improve the hypertension. Relief of any obstruction may also help lower the blood pressure. Nephrolithiasis may occur in 7% to 18% of patients. Secondary infection with Escherichia coli may be seen in 20% to 50% of patients. Genital involvement is common in men with urinary TB. Epididymitis may present with scrotal discomfort, mass, or a cold abscess that may rupture, leading to a nonhealing posterior scrotal sinus. Thickening of the vas deferens may result in the “beaded” vas. TB of the prostate may present with lower urinary symptoms and perineal pain. The prostate may be hard or boggy. Penile and urethral TB may present with strictures, fistulas, ulcers, or papulonecrotic skin lesions. Hemospermia, reduction of semen volume, and infertility are other manifestations of genital involvement. Direct spread of M. tuberculosis to the sexual partner is possible.
Tuberculosis of the Urinary Tract
Definition
Etiology
Pathogenesis
Clinical Manifestations
Clinical Features of Urinary Tuberculosis
Features
Frequency (%)
Symptoms
Asymptomatic
25
Detected during autopsy, surgery, or investigations for other diseases
Asymptomatic urinary abnormalities
25
Persistent pyuria, microscopic abnormalities, hematuria
Lower urinary tract symptoms (most common)
40
Frequency, urgency, dysuria, incontinence, nocturia, suprapubic pain, perineal pain
Male genital tract involvement
75
Epididymitis, hemospermia, infertility, reduced semen volume
Female genital tract involvement
<5
Amenorrhea, infertility, vaginal bleeding, pelvic pain
Constitutional symptoms
<20
Fever, reduced appetite, anorexia, weight loss, night sweats
Miscellaneous
—
Urolithiasis, hypertension, acute kidney injury, chronic kidney disease, abdominal colic, abdominal mass
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tuberculosis of the Urinary Tract
Chapter 54