Transvaginal Ultrasound and the Hysteroscopic Surgeon
Leeber Cohen
Transvaginal ultrasound (TVS) is so widely used today that the merits of this technique do not require extensive review. The study of Padilla et al. clearly demonstrated the severe limitations of bimanual examination in correctly identifying uterine leiomyomata and adnexal masses even under general anesthesia. The sensitivity of TVS for identifying uterine and adnexal pathology is so greatly improved that Goldstein has argued for the routine incorporation of transvaginal ultrasound into annual gynecologic examinations. TVS is also a powerful tool for the evaluation of postmenopausal bleeding. The widely referenced consensus report of the Society of Radiologists in Ultrasound suggests that either transvaginal ultrasound or endometrial biopsy is acceptable for the initial triage of postmenopausal bleeding. TVS with saline infusion by both two- and three-dimensional techniques is a powerful tool for the preoperative mapping of submucous leiomyomata. Three-dimensional TVS can also be used to screen and more accurately describe uterine anomalies. This chapter will review the major applications of TVS as used by the hysteroscopic surgeon.
Abnormal Uterine Bleeding—Premenopausal
Transvaginal Ultrasound without Infusion and Color Doppler Mapping
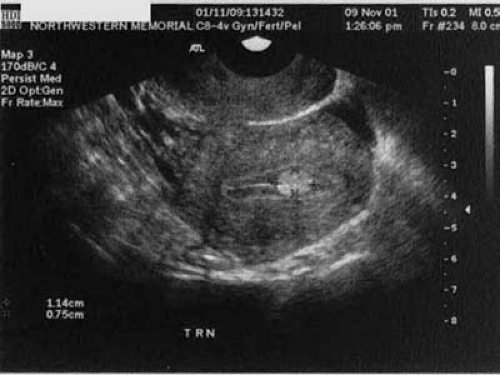
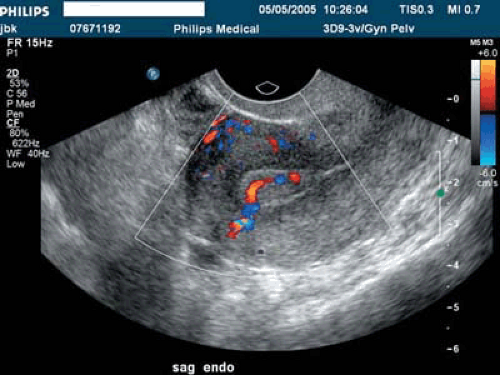
Space-occupying lesions of the endometrial cavity can frequently be identified and mapped by grayscale ultrasound without infusion and the addition of color Doppler. Advantage may be taken of the typical three-line endometrium seen at midcycle (Fig. 19.1). Echogenic endometrial polyps may disrupt the three-line architecture in Figure 19.2. Color Doppler at midcycle can be used to map the subepithelial vessels (Fig. 19.3). This may be useful in the preoperative evaluation of patients with Asherman syndrome. Endometrial polyps and endocervical polyps will frequently display a single central feeder vessel (Figs. 19.4 and 19.5). A feeder vessel may be identified within an echogenic endometrium that is masking an endometrial polyp (Fig. 19.6). A recent study by Alcazar et al. found that TVS with color Doppler without infusion was 95% sensitive and 80% specific for identifying endometrial polyps compared with 100% sensitivity and 80% specificity for sonohysterography. Diagnosis was confirmed with the hysteroscopy. Submucous leiomyomas will frequently display circumferential vessels with multiple perforating vessels (Fig. 19.7A, B). Three-dimensional ultrasound without infusion can frequently be used to map submucous leiomyomas without infusion (Fig. 19.8).
Two-Dimensional Sonohysterography
Many studies are concerned with the diagnostic accuracy of sonohysterography. The 2001 study of Deuholm et al. serves as an excellent review and includes 181 patients with operative correlation with either hysteroscopy or hysterectomy. Although TVS without infusion was very sensitive at 99% in identifying submucous leiomyomas, 21% of endometrial polyps were missed. Further research is required to see if this percentage can be reduced with the addition of color Doppler to TVS without infusion. It is our unpublished experience that many small polyps do reveal a feeder vessel on Doppler investigation and they are easily hidden within an echogenic endometrium (Fig. 19.9). In some cases, the feeder vessel can be seen only after infusion (Fig. 19.10). The clinical significance of these very small polyps is debated.
The technique of sonohysterography is easily learned. Many different catheters have been used for sonohysterography. Our lab uses the Ackrad 7-French Elliptosphere (Marshall Medical) unless the canal is stenotic, in which case a smaller spherical balloon is used. The cervix is prepped with Betadine. The balloon is inserted into the lower uterine segment and filled with 1 to 1.5 mL of air. It is important not to confuse the balloon placed in the lower uterine cavity with a space-occupying lesion. Small dilators or os finders are occasionally required prior to insertion of the catheter. A tenaculum is rarely required. Placement of the elliptical balloon catheter in the cervical canal for better visualization of the complete uterine cavity is also possible if the cervix is not too patulous. The study can usually be performed with 5 to 10 mL of saline if a good seal is maintained with slight
traction on the catheter. Multiple parasagittal and axial cuts should be performed to make sure pathology is not missed. Vagal episodes can occur about 5% of the time. These episodes can be minimized by premedication with ibuprofen or similar drugs, not overinflating the balloon catheter, slow distension of the cavity, and minimizing the volume of injected saline. Exacerbation of pelvic inflammatory disease or pelvic peritonitis is a very rare but known complication. All patients should receive informed consent for sonohysterography.
traction on the catheter. Multiple parasagittal and axial cuts should be performed to make sure pathology is not missed. Vagal episodes can occur about 5% of the time. These episodes can be minimized by premedication with ibuprofen or similar drugs, not overinflating the balloon catheter, slow distension of the cavity, and minimizing the volume of injected saline. Exacerbation of pelvic inflammatory disease or pelvic peritonitis is a very rare but known complication. All patients should receive informed consent for sonohysterography.
 FIGURE 19.1 A three-line endometrium measuring 5 mm in the anterior-posterior (A-P) is noted at midcycle. |
Caution must be taken not to schedule patients for possible sonohysterogram when they may be pregnant. It is our policy to schedule day 5 to 10 of the cycle in women at risk for pregnancy not using hormonal contraception. In women with very irregular cycles, pregnancy testing is ordered if appropriate. TVS of the cervix, uterus, and ovaries without infusion must be performed prior to infusion. An incomplete list of contraindications to proceeding with sonohysterography in our lab includes the finding of hydrosalpinges unless antibiotic prophylaxis has been given, suspected endometrial cancer, identification of an ovarian neoplasm, or identification of recent ovulation (if at risk for pregnancy). Women with known history of pelvic inflammatory disease should receive antibiotic prophylaxis. The finding of a purulent cervical discharge is another obvious contraindication to sonohysterography.
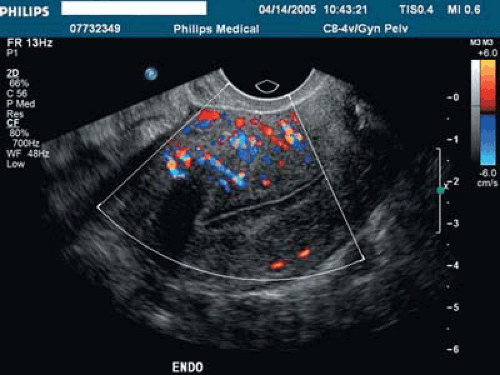 FIGURE 19.3 Subepithelial perforating vessels are noted entering the basalis portion of the endometrium anteriorly. |
Mapping Submucous Leiomyomata
The European Society of Hysteroscopy classification of submucous leiomyomas has received more widespread usage in the United States in the last few years (Fig. 19.11). Although type II leiomyomas that are deeply penetrating into the underlying myometrium can be removed in expert hands, higher complication rates have been noted. In a review by Cohen and Valle, a recommendation was made that less experienced hysteroscopic surgeons should limit themselves to type 0 and type I leiomyomas. A typical 2-D sonohysterogram with power Doppler demonstrating a type II submucous leiomyoma is illustrated in Figure 19.12. The technique of 3-D sonohysterography was described by several authors in the mid-1990s. Our lab has used this technique routinely since 1999. The motorized transvaginal probes make volume acquisition easy and rapid, and the coronal views are immediately reconstructed (Figs. 19.13, 19.14, 19.15, 19.16). The recent publication of Salim et al. compared three-dimensional saline infusion sonohysterography with hysteroscopy and found agreement in 11 of 12 type 0 cases, 34 of 37 type I, and 9 of 12 type II. It must be remembered that MRI with contrast is more accurate in identifying and mapping leiomyomas when more than four leiomyomas are present or uterine volume is >300 mL.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree