Complications of Hysteroscopic Surgery
Michael S. Baggish
Generally, hysteroscopy and hysteroscopic surgeries are safe procedures and are not associated with a high rate of complications. Propst et al. (2000) evaluated complications in 925 women and found that those who had operative hysteroscopic myomectomy and septum resection had greater odds for complications than did those who had polypectomy or ablation procedures; 7.4 and 4.0 versus 0.1 and 0.4, respectively. For convenience, the author has attempted to categorize complications occurring during hysteroscopy. Data about complications are hard to obtain principally because there are no mandatory reporting mechanisms, because surgeons are loath to report complications in the literature, and because of the medicolegal milieu in which most gynecologists work. Aydeniz et al. (2002) evaluated complications in 21,676 hysteroscopic procedures in Germany (92 centers). The rate of complications including anesthesiological was small. Unfortunately, if one performs surgical operations, complications inevitably occur. When an untoward, unexpected adversity happens, the skill of the physician is manifested by the prompt recognition and appropriate management of the complication. Lawsuits invariably follow significant complications. Defense against medical malpractice suits should begin before the operation takes place and, in fact, before it is scheduled. Jansen et al. (2000) reported 38 complications in 13,600 hysteroscopies (0.28%). Diagnostic hysteroscopies had a lower rate of complications than operative hysteroscopy, 0.13% versus 0.95%, respectively. Perforation was the most frequent surgical complication (0.76%). Half the complications were entry related and the other half related to the surgeon’s experience and skill.
Infection
The uterus is particularly resistant to infections because of its ability to regularly shed its epithelial lining. In the author’s experience with 5,000 hysteroscopies, only 13 infections have been documented (nine endometritis and four salpingitis). The latter includes three women who had pyrexia or infection out of 568 ablations reported by Baggish and Sze. Others have reported varying rates of infection with smaller series: Garry et al. (1995) reported only two infections in 600 laser ablations. McCausland et al. studied 200 patients and reported three cases of tubo-ovarian abscess. Salat-Baroux et al. reported seven mild infections out of
4,000 hysteroscopic examinations. Some 90% of these were performed in an office setting.
4,000 hysteroscopic examinations. Some 90% of these were performed in an office setting.
Although soaking in Cidex (2% glutaraldehyde) had been a standard for sterilization in the past, gas sterilization (ethylene oxide) or steam autoclaving should be used in both hospital and out-of-hospital settings whenever possible. Nevertheless, according to an Emergency Care Research Institute (ECRI) report of 1994, no study has conclusively demonstrated that high-level disinfection (e.g., liquid chemical germicide) poses an infection risk. Agostini et al. (2002) studied 1,952 patients over 10 years having a total of 2,116 operative hysteroscopies. Thirty infections were recorded and consisted of 18 (0.85%) cases of endometritis and 12 urinary tract infections. No severe infection occurred. Santos et al. (2004) reported on serum and hysteroscopic samples taken in 62 women after diagnostic hysteroscopy. At least one hepatitis B virus (HBV) marker was found in 49 (79%) samples. Although HBV-DNA could be identified on the hysteroscope after hysteroscopy, standard disinfecting techniques were effective in removing the virus. The latter included soaking in glutaraldehyde.
Surgeon Related
Most injuries attributed to surgical technique are the result of lack of experience, unfamiliarity with equipment, insufficient anatomic knowledge, or lack of skill. The greatest problem concerns level of experience. A report from the Swedish Hospital Medical Center by Smith et al. reviewed all endoscopic procedures relative to outcome and possible hazards. Over a 15-month period, 42 physicians performed 227 procedures. A total of 100 operative hysteroscopies were done, and 25 complications were recorded. Some 50% of the gynecologists performed three or fewer procedures, and 33% performed five or more procedures. One surgeon accounted for 15% of the total.
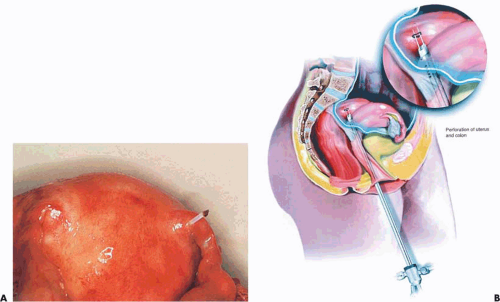
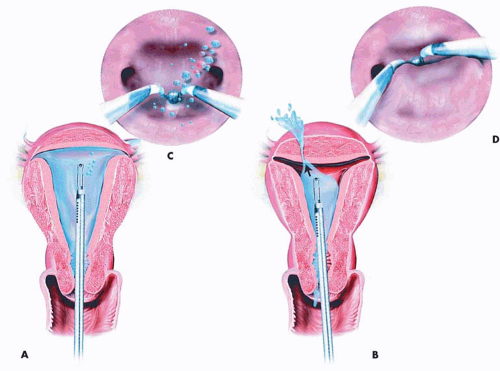
Perforation of the uterus can occur with any hysteroscopic operation (Fig. 30.1). This is the principal reason that a simultaneous laparoscopy is performed during septum incision surgery. In the worst-case scenario, perforation with scissors or curette may result in bleeding, which can usually be controlled by suture placement. When perforation occurs with a laser fiber or electrosurgical device, the risk of injury to structures anatomically close to the uterus is a real possibility (Fig. 30.2A, B). Therefore, these structures (e.g., bowel, bladder, ureters, great vessels) should be explored to rule out an associated injury. Furthermore, the operator should have been taught never to apply power to a laser fiber or electrode while advancing the instrument. Power is applied only when the device is being pulled downward, i.e., returning to the sheath (Fig. 30.3).
Perforation is signaled by inability to maintain proper distention of the cavity because the medium is leaking out through the hole in the uterus (Fig. 30.4). Additionally, an astute observer will note the medium flow exiting from a site other than the normal exits (i.e., the tubal ostia).
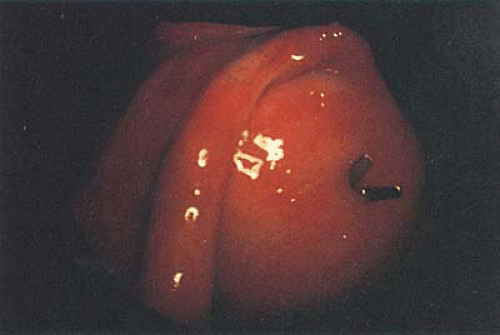 FIGURE 30.1 Uterine perforation caused by hysteroscopic scissors. Generally, minimal damage is created when perforation occurs with the mechanical device. |
Interestingly, in two large series of endometrial ablations totaling approximately 1,200 cases, no perforations were reported.
Comparing methods for performing endometrial ablation, the Royal College of Obstetricians and Gynaecologists Audit Unit Mistletoe (minimally invasive surgical technique laser endothermal, or endoresection) study of >7,534 cases found that laser and ball electrode ablation were significantly safer than endometrial resection. The latter uses a loop electrode to cut strips of endometrium. Table 30.1 shows the comparative complication rate.
In 1995, data from the Scottish Hysteroscopy Audit Group reported on 978 hysteroscopic procedures (629 resections, 314 laser ablations, 35 roller-balls) based on consultant and senior registrar registration of cases. A total of 120 complications (12%) were reported, including 61 cases of fluid overload; 11 perforations, of which 6 were via resectoscope; and 35 cases of excessive bleeding. A total of three hysterectomies were performed for operative complications; one death occurred secondary to septicemia.
TABLE 30.1 Complications Associated with Endometrial Ablation or Resection Based on 7,534 Cases | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Following a hysteroscopic operation, pain is typically of a cramping nature and diminishes as the length of time from
the day of operation increases. Severe abdominal pain, nausea, vomiting, and fever are distinctly abnormal and should alert the gynecologist to first look for a problem related to the surgical procedure. The finding of free air within the peritoneal cavity means bowel perforation until proven otherwise (Fig. 30.5).
the day of operation increases. Severe abdominal pain, nausea, vomiting, and fever are distinctly abnormal and should alert the gynecologist to first look for a problem related to the surgical procedure. The finding of free air within the peritoneal cavity means bowel perforation until proven otherwise (Fig. 30.5).
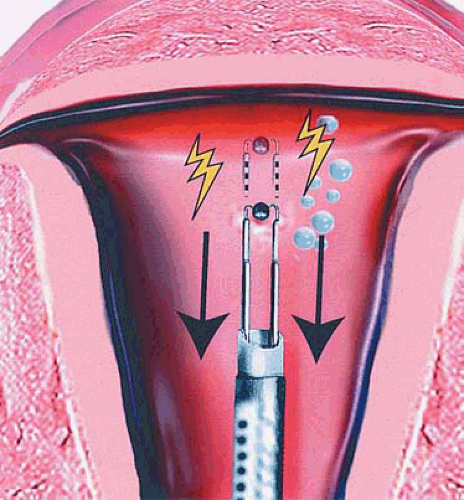 FIGURE 30.3 The operator should never apply power to an electrosurgical device while advancing the electrode. The power can safely be applied as the electrode returns toward the sheath. |
Uterine rupture may occur in a subsequent pregnancy after hysteroscopic perforation or surgery invading the myometrium. Kerimis et al. (2002) reported a fundal rupture requiring emergency cesarean section in a 37-year-old woman who underwent prior septum resection using hysteroscopic electrosurgery. Angell et al. (2002) reported a similar case in which scissors were used to cut the septum. In both cases, the rupture was transverse along the fundus, i.e., cornua to cornua. Gabnele et al. (1999) reported uterine rupture requiring immediate cesarean section in a patient who had previously undergone a hysteroscopic metroplasty. In this case, the labor was induced with Prostaglandin E2. An unusual case of a uterine fistula in a previously embolized uterus where a myoma migrated through the uterine wall was recently reported. The fistula occurred following the hysteroscopic removal of the migrated myoma. Several reports of subsequent uterine rupture have been documented in the literature following myomectomy, perforation during entry, or during surgery. Therefore, when any of the above events
occurs, it is incumbent for the attending surgeon to explain to the patient about the risk of uterine rupture in a subsequent pregnancy and to document this discussion clearly in the medical record. Additionally, the subsequent obstetrician should likewise apprise the patient of the risk of rupture. The author believes the interval between uterine operations that infringe on the myometrium and attempts for pregnancy should not be less than 1 year from the date of the uterine surgery.
occurs, it is incumbent for the attending surgeon to explain to the patient about the risk of uterine rupture in a subsequent pregnancy and to document this discussion clearly in the medical record. Additionally, the subsequent obstetrician should likewise apprise the patient of the risk of rupture. The author believes the interval between uterine operations that infringe on the myometrium and attempts for pregnancy should not be less than 1 year from the date of the uterine surgery.
Media Related
Some of the data on complications of intrauterine infusion of gas or liquids have been discussed in Chapter 16 on media.
CO2 embolism may occur during diagnostic hysteroscopy. This is more likely to happen when a proper CO2 hysteroscopic insufflator is not used. Nevertheless, even with an appropriate insufflator, embolism can occur. Brink et al. reported such a case and emphasized that the diagnosis of a large CO2 embolism is suggested by rapid fall in expired CO2 concentration, as well as the presence of mill wheel murmur.
Brundin and Thomasson observed 70 women during CO2 hysteroscopy. Seven women (10%) developed “typical metallic heart sounds” during the hysteroscopy. The hysteroscopy was immediately discontinued, and the abnormal heart sounds disappeared. They recommended that cardiac auscultation be performed during CO2 hysteroscopy. Corson et al. (1988) experimented on direct vascular insufflation of CO2 gas into anesthetized ewes. CO2 flow rates ranged from 30 to 90 mL per minute. Po2 showed no significant change other than a dip at 20 minutes with a flow of 60 mL per minute. The Pco2 levels were elevated only at 90 mL per minute. The lowest pH reading was 7.27. Only one electrocardiogram (ECG) tracing showed a significant abnormality. The authors concluded that CO2 clearance is very efficient and tolerance to intravascular CO2 is high because of its solubility in blood. They did caution that patients with septal defects and pulmonary hypertension were at risk when this agent is used for uterine distention.
Air embolism is a definite risk during hysteroscopy. Several cases of death or significant neurologic injury have been associated with embolized air introduced into the uterus by sapphire-tipped laser fibers and coaxial laser fibers
(Figs. 30.6 and 30.7). Similarly, Perry and Baughman reported two cases of air embolism associated with hysteroscopy. In 1996, Corson et al. reported four cases of catastrophic air embolism associated with hysteroscopy. The Trendelenburg position should not be used during hysteroscopy, and all tubing, as well as the hysteroscope, should be purged of air before inserting it into the uterus. Similarly, careful dilatation should be done to avoid opening up venous channels (Fig. 30.8). Bloomstone et al. (2002) reported ten cases in which gas bubbles were detected in the hepatic veins or right heart in ten patients who had monopolar resectoscopic surgery. The authors hypothesized the gas bubbles may have emanated from the destruction of cells and release of gas bubbles via the action of the electrosurgery. Imasogie et al. (2002) reported the case of a 50-year-old woman who underwent endometrial ablation and resection in the Trendelenburg position who suddenly developed oxygen desaturation (97% to 87%) and drop in end tidal CO2 (46 mm to 27 mm). The authors hypothesized gas embolism secondary to the products of resectoscopic combustion.
(Figs. 30.6 and 30.7). Similarly, Perry and Baughman reported two cases of air embolism associated with hysteroscopy. In 1996, Corson et al. reported four cases of catastrophic air embolism associated with hysteroscopy. The Trendelenburg position should not be used during hysteroscopy, and all tubing, as well as the hysteroscope, should be purged of air before inserting it into the uterus. Similarly, careful dilatation should be done to avoid opening up venous channels (Fig. 30.8). Bloomstone et al. (2002) reported ten cases in which gas bubbles were detected in the hepatic veins or right heart in ten patients who had monopolar resectoscopic surgery. The authors hypothesized the gas bubbles may have emanated from the destruction of cells and release of gas bubbles via the action of the electrosurgery. Imasogie et al. (2002) reported the case of a 50-year-old woman who underwent endometrial ablation and resection in the Trendelenburg position who suddenly developed oxygen desaturation (97% to 87%) and drop in end tidal CO2 (46 mm to 27 mm). The authors hypothesized gas embolism secondary to the products of resectoscopic combustion.
 FIGURE 30.5 Abdominal upright film shows free air present beneath the diaphragm. This typifies injury to a hollow viscus such as the large or small bowel. |
 FIGURE 30.6 Numerous shapes of sapphire tips deployed in conjunction with a coaxial fiber. The coaxial fiber allows cooling of the tips, usually with air or nitrogen gas. |
 FIGURE 30.7 In contrast to the coaxial fiber, a bare fiber is illustrated here. No cooling is required, and therefore no air can be introduced into the uterine cavity via this fiber. |
 FIGURE 30.8 Careful dilatation is best done with Pratt dilators. The patient should not be placed in the Trendelenburg position during any phase of the hysteroscopy. |
Two types of complications have been reported with the use of 32% dextran 70 (Hyskon) as the uterine distention medium: anaphylaxis and Hyskon reaction. The anaphylactoid reaction is rare and is related to a typical allergic response characterized by wheal formation, rash, itching, and laryngeal edema. This occurs because the dextran molecule is a complex of branched polysaccharides. Seven cases of a true Hyskon reaction have been reported in the literature. Many of these cases hypothesize the mechanism of noncardiogenic pulmonary edema, which was originally proposed in a nongynecologic case report authored by Kaplan and Sabin. Curiously, this mechanism was suggested notwithstanding the absence of central venous pressure and pulmonary wedge pressure measurements. A similar mechanism was cloned from the Kaplan and Sabin paper by Zbella et al., Leake et al., and Mangar et al., but corrected by Lukacsko. In reality, the Hyskon reaction can be very well explained based on the exaggerated physiologic actions of dextran. Pulmonary edema results from the intravascular uptake of dextran 70. As noted in the chapter an infusion media, dextran is osmotically active and pulls extracellular fluid into the vascular space. When the volume of fluid entering the vascular space doubles or trebles the plasma volume, pulmonary edema may occur but is dependent on the ability of the patient’s heart to effectively pump the increased load (Fig. 30.9A–D).
Renal failure occurs because dextran 70 is a mixture of macromolecules, which can produce elevated plasma oncotic pressures. According to Moran and Kapsner, the hyperoncotic state (i.e., when oncotic forces equal or exceed the hydraulic forces) causes glomerular filtration to cease. Dextran also can precipitate within renal tubules, forming occlusive casts. The treatment for the above problems is plasmapheresis.
The bleeding diathesis associated with elevated levels of Hyskon in the blood stream can be clearly explained based on the therapeutic actions of dextran on the clotting mechanism. The major effect is three pronged:
Steric exclusion of fibrinogen
Reduction of factor VIII R (von Willebrand factor)
Decreased platelet adhesiveness and aggregation
Ruiz and Neuwirth reviewed data on 1,793 patients who had hysteroscopy in which Hyskon was the distention medium. Eighteen complications were observed, of which possibly three were thought to be Hyskon related. One complication involved only a rash on the thorax and abdomen. The other two patients developed pulmonary edema (0.11% incidence), with one of the two also developing disseminated intravascular coagulation (DIC) (0.05%). The volume of Hyskon in the two serious cases were 650 and 700 mL, respectively. The authors recommended not administering >500 mL of Hyskon at any one time during hysteroscopic surgery.
Baggish et al. (1992) studied the vascular uptake of Hyskon during operative and diagnostic hysteroscopy. The volume of Hyskon instilled, Hyskon blood levels, intrauterine pressure, plasma osmolality, sodium and potassium levels, coagulation factors, and operative times were measured. Blood samples were obtained before, during, and after hysteroscopic surgery. The volume of Hyskon instilled ranged from 7 to 550 mL. No correlation could be established between the volume of Hyskon instilled into the uterus and Hyskon blood levels (e.g., when 550 mL was instilled, the Hyskon blood level was 800 mg %, whereas when 260 mL was instilled, the blood level was 3,000 mg %) (Tables 30.2 and 30.3). The data showed that damage to the endometrium and/or myometrium was related to elevated Hyskon blood levels. The highest levels were seen in endometrial ablation and uterine synechiae (2,900 to 3,400 mg %). Significant reductions (preoperative vs. postoperative) were noted in serum K and fibrinogen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree