Barbara A. Greco, Jamie P. Dwyer, Julia B. Lewis
Thromboembolic Renovascular Disease
Renal arterial and venous thromboembolic disease presents as a wide variety of clinical syndromes, ranging from vascular catastrophe with acute renal infarction to progressive decline in renal function as a result of chronic renal ischemia. Appropriate imaging allows accurate and timely identification of renal infarction, renal artery or venous thrombosis, and other renovascular abnormalities.
Thrombotic microangiopathies are discussed in Chapter 29, and renovascular hypertension and atherosclerotic ischemic renal disease are discussed in Chapter 39.
Normal Anatomy
Renal Artery
The anatomic considerations that affect the clinical presentations and outcome of renal arterial thromboembolic events include the size of the vessel involved, from the main renal artery and the branch vessels to the arterioles and glomerular capillaries, and the condition of the collateral blood supply. In most individuals, the kidney has a single artery with a diameter of 3 to 7 mm. The main renal artery branches in either a double or a triple fork pattern or, less commonly, a ladder pattern.1 The incidence of multiple renal arteries is about 30% with bilateral supernumerary arteries in 10%. The right renal artery usually passes posterior to the inferior vena cava (IVC), but rarely can be precaval.
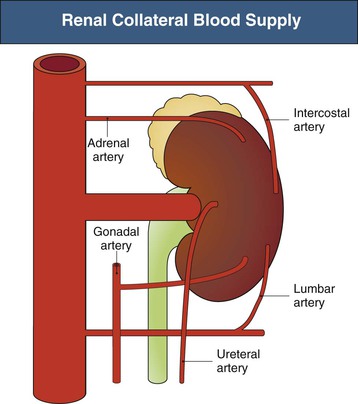
The main collateral vessels to the kidney include the suprarenal, the lumbar, and the ureteral vessel complexes, which can maintain renal parenchymal viability in the face of main renal arterial occlusion. The collateral circulation to the kidney is depicted in Figure 66-1. In a study examining 301 aortograms, the adrenal arteries supplied collateral circulation to the kidney in 60% of cases, the lumbar in 55%, the ureteric in 15%, and the gonadal in 13%.2 The factors determining the development and caliber of these vessels are poorly understood but include individual anatomy, state of the aorta, rate of progression of main renal artery narrowing, and condition of the intrarenal perforating arteries.
Renal Vein
Renal veins begin in the subcapsular region of the kidney. These stellate veins communicate with perirenal and cortical venous channels and empty into interlobular veins that drain into arcuate veins. The venae rectae drain the pyramids and join the arcuate veins. Arcuate veins leave the renal parenchyma through interlobar vessels, converging into four to six trunks near the hilum of the kidney (Fig. 66-2). Two or more renal veins have been described in up to 20% of people; this is more common on the right.3 The main renal veins empty into the IVC; the left renal vein is three times longer than the right (7.5 cm vs. 2.5 cm). The left renal vein traverses behind the splenic vein and body of the pancreas before it crosses in front of the aorta near its termination in the IVC. Anatomic variation exists such that up to 2.3% of people have retroaortic renal veins and under 1% have circumaortic veins with branches extending both behind and in front of the aorta.4 The left renal vein collects the flow from the left ureteral, gonadal, adrenal, and inferior phrenic veins. In Gerota’s fascia, the perirenal venous network communicates with the retroperitoneal collateral veins from the lumbar, azygos, and tributaries of the portal system.5,6
Thromboembolic Renovascular Disease
Thromboembolic renovascular disease results in disruption of normal renal blood flow, which may lead to either renal ischemia or overt infarction. The caliber and type of vessels involved (artery, arteriole, vein) as well as whether one or both kidneys are affected determine the clinical presentation (Box 66-1).
Thromboembolic Ischemic Renal Disease
Ischemic renal disease caused by atherosclerotic renovascular disease (ASRVD) is discussed in Chapter 39. On occasion, thrombosis of the renal artery or branch vessels occurs acutely and without overt renal infarction, providing a clinical opportunity for renal salvage. The clinical settings in which this might occur include spontaneous thrombosis of atherosclerotic renal artery occlusive disease, spontaneous dissection of the aorta involving the origin of the renal artery, dissection of the renal artery itself, and as a consequence of aortic or renal artery interventions including endovascular stenting.
The clinical presentation of renal artery or branch vessel thrombosis without infarction often involves acceleration of hypertension because of renin release, worsening renal function, and even anuria if the entire functioning renal mass is involved. Patients may develop volume overload, pulmonary edema, and symptoms of uremia.
The clinical evaluation of the patient includes ruling out overt renal infarction and defining renal parenchymal viability despite main renal or branch vessel thrombosis. This can be achieved by demonstrating a nephrogram on delayed venous-phase imaging by conventional renal arteriography, computed tomographic angiography (CTA) or contrast-enhanced computed tomography (CT), magnetic resonance angiography (MRA) with gadolinium enhancement, or nuclear isotope scintigraphy. The diagnostic approach is complicated in the setting of reduced renal function because of the concern of nephrotoxicity from radiocontrast dye. In addition, the use of gadolinium with MRA is contraindicated if glomerular filtration rate (GFR) is below 30 ml/min because of the risk of nephrogenic systemic fibrosis.
Treatment involves assessment of the risks versus potential benefits of heroic revascularization procedures. The status of the contralateral kidney and overall residual renal function (RRF) should be weighed against the potential retrievable renal function from the underperfused kidney. The size and baseline function of the kidney before the thromboembolic event influence the potential benefits of renal revascularization. The cardiac and anesthesia risk from the intervention, such as surgical thrombectomy or bypass, has to be considered. Last, the intervention should be delayed until the clinical and renal status of the patient has recovered from any transient insults, such as acute kidney injury (AKI), decompensated congestive heart failure (CHF), myocardial infarction, and uncontrolled hypertension.
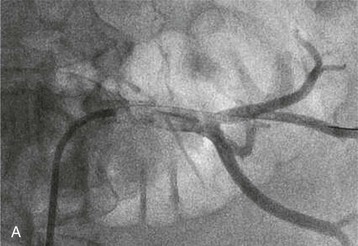
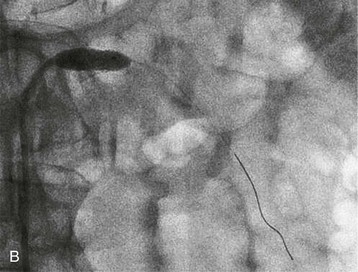
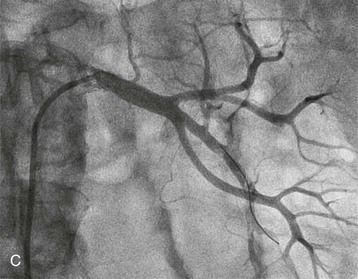
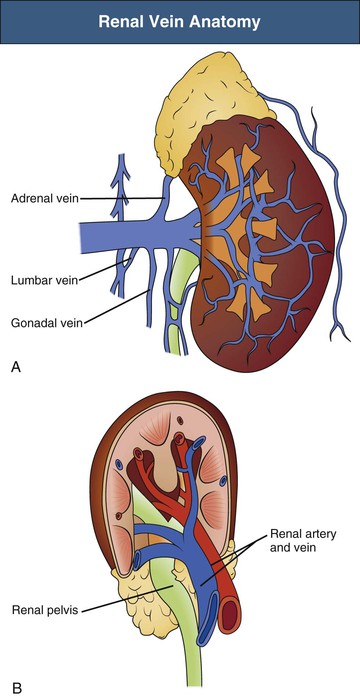
Renal artery revascularization can be achieved by endovascular thrombolysis, angioplasty, and stenting or by surgical approaches. This can be successful in restoring renal function even in patients with dialysis-dependent renal failure caused by occlusive renovascular disease.7–9 Predictors of renal recovery are listed in Box 66-2 and include preserved renal size, evidence of a renal “blush” or nephrogram by imaging, recent decline in GFR, and recent baseline creatinine concentration below 3 mg/dl.10 Figure 66-3 shows thrombosis of a renal artery within a stent placed 1.5 years previously for atherosclerotic renal artery stenosis in a patient with a single functioning kidney. The patient presented with anuric AKI. Percutaneous thrombolytic therapy and renal artery angioplasty restored renal perfusion and renal function.
Renal Infarction
When abrupt interruption of renal blood flow occurs without adequate collateral blood supply, renal infarction may occur. Autopsy series suggest the incidence is between 0.5% and 1.5%. Renal infarction may involve the entire kidney or small areas of the cortex or medulla. Both arterial and venous thrombosis and renal artery embolism can cause renal infarction. The most common presenting symptoms of renal infarction include loin, flank, or abdominal pain with nausea and/or vomiting. Urinalysis often demonstrates microscopic hematuria and proteinuria. Transient or accelerated hypertension may occur secondary to abrupt release of renin from the infarcted segment. Up to one quarter of cases are asymptomatic, identified only by enhancement or functional defects on renal imaging. Transient elevations in serum creatinine are not uncommon. When bilateral occlusion of both kidney arteries or infarction of a single functioning kidney occurs, the patient presents with oliguric or anuric AKI. Systemic signs of renal infarction include leukocytosis and fever. Tissue injury results in elevations of serum enzymes, most commonly lactate dehydrogenase, but transaminases, creatine kinase, and alkaline phosphatase may also be elevated.12,13
Diagnosis
Because the presenting symptoms are common to a number of disorders, a high clinical suspicion is required for the diagnosis of renal infarction. CT with intravenous contrast administration is the imaging modality of choice for demonstrating areas of the renal cortex that are not perfused. CT may demonstrate focal wedge-shaped areas of decreased attenuation or global infarction with or without a rim sign indicating intact collateral circulation to the renal cortex.14 Perinephric stranding may be present as well. It is not uncommon to see simultaneous infarcts in the spleen or liver. Other potential imaging techniques include MRA with gadolinium (if GFR remains above 30 ml/min) or as time-of-flight or phase-contrast imaging, or nuclear scintigraphy with dimercaptosuccinic acid (DMSA) (Fig. 66-4). When the entire kidney is underperfused, it is often difficult to determine whether there may be salvageable renal parenchyma. Studies in experimental animals with acute renal artery occlusion have shown that the collateral circulation can maintain renal viability for up to 3 hours after occlusion.15 In patients with underlying ASRVD, collateral vessels may be better developed and renal viability may be maintained for days to weeks. In these patients, urgent arteriography to identify the location of the arterial thrombosis or embolus may allow percutaneous or surgical revascularization.
Etiology
The most common causes of renal infarction are trauma, renal artery embolism from cardiac thrombus, and iatrogenic complications of endovascular procedures (Box 66-3). Spontaneous renal artery thrombosis or dissection is most often associated with atherosclerotic disease of the aortic or renal arteries, but other vascular causes include fibromuscular dysplasia, Takayasu arteritis, polyarteritis nodosa, Marfan syndrome, and Ehlers-Danlos syndrome with associated dissection or aneurysms. Less common causes of infarction include hypercoagulable states, most commonly nephrotic and antiphospholipid antibody syndromes; inflammatory diseases of the retroperitoneum or aortorenal vasculature; and thrombotic microangiopathies (TMAs).
Thrombosis Caused by Trauma
Traumatic injuries to the renal arteries make up 1% to 4% of all nonpenetrating abdominal trauma. Classically, kidney trauma results from a deceleration injury, such as a fall from a great height with an upright landing on impact. This results in stretching of the renal arteries as the kidneys continue downward after the rest of the body has stopped. The subsequent stretching and recoiling of the renal arteries can result in acute thrombosis, which is typically bilateral. Direct blunt trauma to the loin or flank regions associated with motor vehicle accidents, street fights, and sports injuries can also result in renal artery thrombosis.
Evidence of lumbar vertebral injury should raise suspicion in the emergency department for renovascular trauma. Even when this injury is diagnosed early, the success rate for renal revascularization after trauma to the renal vessels remains low, between 0% and 29%.16 The recently revised renal injury scale classification defines a class V renal injury as either main renal artery or vein laceration, avulsion, or thrombosis. In a series of 3580 traumatic renal injuries from a single institution, class V injuries had a renal salvage rate of only 4.3% and a high nephrectomy rate of 74%.17
Injuries to the renal pedicle that result in diminished perfusion to a single functioning kidney or to both kidneys require rapid intervention, and endovascular stabilization of renal blood flow may be helpful as a bridge to more definitive renal revascularization.
Thrombosis Caused by Hypercoagulable Disorders
Clotting disorders, such as protein C or protein S deficiency, antithrombin III deficiency, elevations in factor VIII, and rarely factor V Leiden mutations, can predispose to renal arterial thrombosis and infarction in addition to their association with renal vein thrombosis (RVT). Hypovolemia, polycythemia, and the use of oral contraceptive agents can increase the risk of thrombosis when these underlying disorders are present.
Antiphospholipid antibody syndrome is associated with both arterial and venous thrombotic events and can involve the renal circulation at any level. In patients younger than 50 years, the antiphospholipid syndrome can account for 15% to 20% of all deep venous thromboses and 30% of strokes. It is the most common cause of spontaneous arterial thrombosis. In one report of 16 cases of renal involvement with this syndrome, 15 of 16 patients had either arterial or venous thrombosis, 10 had intrarenal microangiopathy, one had suprarenal aortic occlusion, and one had main renal artery thrombosis.18 Concomitant thromboses in the mesenteric and cerebral circulation have been reported.19,20
Renal Artery Embolism
The kidneys are frequently the target for emboli from thrombi originating in the heart. In one series, 1.4% of the general population had renal artery embolism at autopsy, of which only 2 of 205 cases (1%) were diagnosed clinically.21 The prevalences of left and right renal emboli are equal, and 12% of cases are bilateral.22 Atrial fibrillation, cardiac thrombus after myocardial infarction, atrial myxoma or other cardiac tumors, endocarditis, paradoxical emboli, and aortic thrombus represent most of the conditions associated with renal embolism. Atrial fibrillation is the most common cause, with a rate of embolism four times higher than that of the general population; the highest risk is during the first year after the diagnosis of atrial fibrillation, when anticoagulation is subtherapeutic. When echocardiography is undertaken, cardiac thrombus is only rarely detected. Other causes of renal emboli include fiber or foam related to cardiac bypass procedures, calcium from valve annuli, and even “bullet emboli” in the setting of trauma. Aortic endovascular stenting has been associated with a 10% incidence of new renal infarcts, presumably of embolic origin.23
Paradoxical renal artery embolism may occur in patients with right-to-left cardiac shunts. The most common cardiac shunt is a result of atrial septal defects, which are present in up to 9% to 35% of the general population. The diagnosis of paradoxical embolism requires clinical, angiographic, or pathologic evidence of systemic embolism and the presence of venous thrombus along with an abnormal communication between the right and left circulations and a favorable pressure gradient (typically diagnosed by “bubble” echocardiography) for the passage of clot from the right to the left side of the heart.
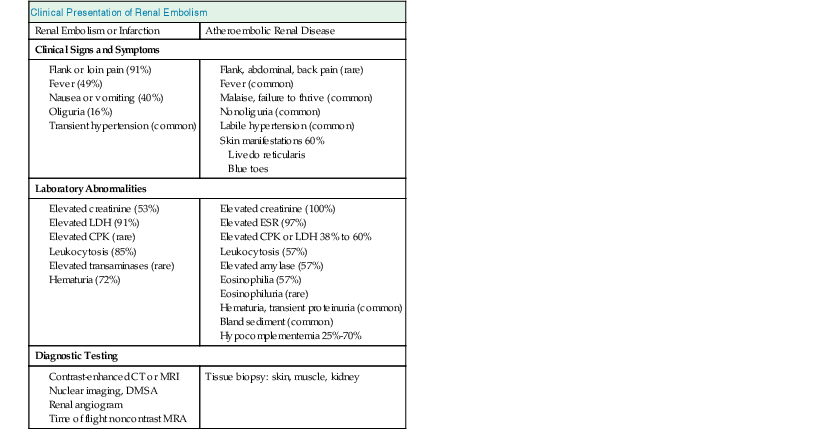
The clinical presentations of renal artery embolism mirror those of renal infarction (Table 66-1).22 Pain is present in more than 90% of cases confirmed by radiologic imaging techniques. It is unclear from the literature how many go undiagnosed because of lack of clinical symptoms. There is a 30-day mortality rate of 10% to 13% among patients experiencing renal embolism in the setting of atrial fibrillation.24 Up to 40% of cases have at least transient reduction in renal function.
Table 66-1
Clinical presentation of renal embolic disease.
CPK, Creatine phosphokinase; CT, computed tomography; DMSA, dimercaptosuccinic acid; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
| Clinical Presentation of Renal Embolism | |
| Renal Embolism or Infarction | Atheroembolic Renal Disease |
| Clinical Signs and Symptoms | |
| Laboratory Abnormalities | |
| Diagnostic Testing | |
| Tissue biopsy: skin, muscle, kidney | |
(Modified from reference 22.)
Although angiography is the gold standard for diagnosis of renal artery embolism, nuclear isotope scanning is also very sensitive (97%). Contrast-enhanced CT detects 80% of renal embolic events.22 In other reports, CT or CTA has a sensitivity nearly matching that of renal angiography.
Aortic and Renal Artery Dissection
Aortic dissection can involve the origin of either renal artery, with the false lumen occluding the vessel and impairing renal perfusion. In this setting the predictors of renal salvage are the same as those for occlusion caused by atherosclerotic renal artery stenosis and include preserved renal size, collateral circulation permitting renal viability, and blush on imaging studies. In one report, despite renal atrophy, aortic stent graft placement allowed renal reperfusion and restoration of renal size and function.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree