The Normal and Diseased Kidney in Pregnancy
Michelle A. Hladunewich
Ayodele Odutayo
Ravi Thadhani
Of all medical disorders that add risk to pregnancy, renal disease is ranked among one of the most feared by physicians. Kidney disease during pregnancy, even when mild, can considerably increase both the maternal and fetal risk necessitating close follow-up by both specialists in nephrology and high-risk obstetrics. Risk increases with the degree of renal dysfunction and is further heightened by comorbid conditions like diabetes and hypertension, which are often now present for many years given the societal trends in developed countries to delay childbearing. In addition to advanced maternal age, risk is further exacerbated by the expanded use of reproductive technologies that often results in multigestational births. Thus, the role of the nephrologist must begin prior to conception to stabilize a woman’s condition and provide an appropriate risk assessment. During pregnancy, close monitoring by both subspecialties likely improves outcome by providing early identification of both renal and fetal compromise.
The goal of this chapter is to provide practical clinical guidance to physicians consulted when such patients contemplate conceiving or are already pregnant. First, we briefly review the normal gestational anatomic and physiologic renal changes, as such knowledge permits early detection of abnormalities. We subsequently discuss an approach to risk stratification and optimization during prepregnancy counselling and, finally, management of renal disease in pregnancy including acute compromise secondary to preeclampsia and other causes of acute kidney injury specific to pregnancy.
PREGNANCY-INDUCED CHANGES IN RENAL ANATOMY AND FUNCTION
Anatomy
It is classically taught that kidney size increases in a normal human pregnancy likely due to a combination of increased renal weight, dilatation of the renal collecting system, and perhaps increased glomerular volume. Contemporary data utilizing imaging techniques or the assessment of human biopsy or autopsy tissue carefully delineating healthy renal accommodation to pregnancy, however, are truly limited. Older data that included 97 women dying during or shortly after pregnancy from causes other than preeclampsia suggested that the increased combined renal weight of “normal” kidneys to be greater than in nonpregnant women, but data for age-matched nonpregnant females was not presented.1
Radiologic estimation of kidney length shows an approximate 1-cm increase in renal size and notes dilatation of the collecting system—calyces, renal pelvis, and ureters— that is significantly more pronounced on the right side (Fig. 59.1).2,3 In the largest study in the literature wherein over 1,000 women were followed serially during pregnancy, dilatation in the right kidney began in the sixth week of gestation and maximal dilatation progressed at a rate of 0.5 mm per week until week 24 to 26 and then slowed to 0.3 mm per week until term.4 Dilatation on the left was less marked as in earlier studies. Although the majority of gravid women (>50%) demonstrate some degree of dilatation,4,5,6 there is significant variability between patients and even serial variability within the same patient making the diagnosis of true obstruction challenging.7 Resolution begins immediately postpartum, but return to the prepregnancy state likely takes a number of weeks.6
The mechanism of this dilatation of the urinary tract is not entirely clear and is most likely a combination of pregnancy-related hormonal factors and mild obstruction. Support for hormonal factors includes the evidence of early dilatation before the uterus has enlarged sufficiently to become an obstructive factor as well as persistence into the postpartum period after delivery.4,6 One study, however, found no correlation between dilatation and either serum estradiol, serum progesterone, or urinary estradiol excretion, but perhaps other hormonal factors that have not been studied in relation to dilatation may be important.5 The best evidence for the obstructive theory comes from studies in which intraureteral pressure was monitored in third trimester gravid patients utilizing a fluid-filled catheter connected to a strain gauge transducer.8 Pressure was greatest in the supine or standing position, but decreased markedly in a lateral decubitus or knee-to-chest position as well as immediately
after cesarian delivery of the fetus, implying that the gravid uterus can obstruct the ureters. In addition, the increased pressure was present only above the pelvic brim where the ureters and the iliac arteries cross. Although this is consistent with the dilatation pattern in most gravid women,9 the largest study to date did note at least some degree of dilatation in 10% to 15% of women in both kidneys before the uterus reached the pelvic rim. Thus, a combination of hormonal factors as well as obstruction is likely of physiologic importance.
after cesarian delivery of the fetus, implying that the gravid uterus can obstruct the ureters. In addition, the increased pressure was present only above the pelvic brim where the ureters and the iliac arteries cross. Although this is consistent with the dilatation pattern in most gravid women,9 the largest study to date did note at least some degree of dilatation in 10% to 15% of women in both kidneys before the uterus reached the pelvic rim. Thus, a combination of hormonal factors as well as obstruction is likely of physiologic importance.
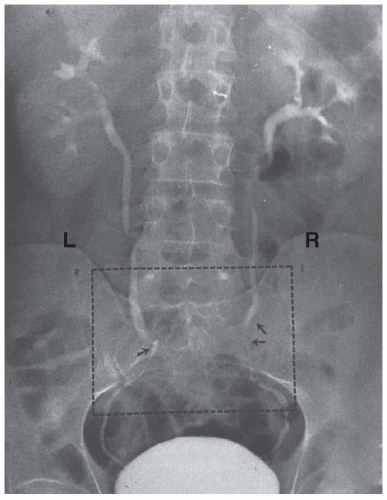 FIGURE 59.1 Normal physiologic dilatation of the urinary collecting system. Note the increased dilatation on the right side. |
In healthy pregnant subjects, the anatomic changes that occur at the level of the glomerulus are even less well described. There is data from 27 autopsy cases1 and more recently from 12 third trimester biopsies10 to suggest that glomerular diameter is greater than that measured in nonpregnant subjects. As already mentioned, the autopsy series conducted within 2 hours of death by the celebrated pathologist H.L. Sheehan between 1935 and 1946 at the Glasgow Royal Maternity Hospital did not include age-matched nonpregnant controls, but instead compared their data to even older autopsy studies wherein the timing and effect of autolysis was not clearly described.1 The more recent biopsy series also failed to utilize careful stereologic techniques to carefully assess the glomerulus, but noted endotheliosis, albeit to a lesser degree, in healthy pregnant controls as well as in patients with preeclampsia.
Renal Function
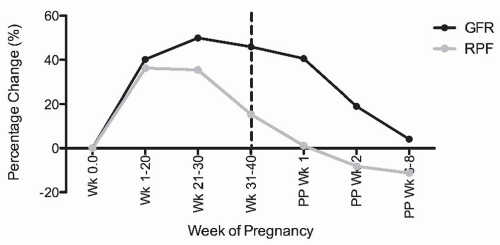
Healthy pregnant women exhibit marked hyperfiltration. Both the glomerular filtration rate (GFR) and renal plasma flow (RPF) increase markedly during gestation. Unfortunately, however, there are few studies in which inulin or iothalamate and p-aminohippurate (PAH) clearance were measured serially and simultaneously throughout gestation in a sizable study population. Therefore, this knowledge comes from a synthesis of a small number of available studies that assessed renal physiology with proper clearance methodology at different time points during gestation (Fig. 59.2).
Hyperfiltration begins as early as the sixth week of gestation.11 By the second half of pregnancy, GFR is elevated by
40% to 60% above normal, nongravid levels, whereas the increase in RPF at least during the first trimester surpasses that of GFR with an increase in the order of 50% to as high as 80% in one study.11,12,13,14,15,16,17,18,19 Thus, the filtration fraction is thought to fall in a pattern consistent with renal vasodilation. These high levels are maintained through gestational week 36, after which there is a decrease in GFR and a more pronounced decrease in RPF returning the filtration fraction to normal or a slightly elevated value compared to values observed in nonpregnant populations.17,20,21 Despite normalization of RPF, the GFR remains elevated by approximately 41% on the first postpartum day20 and by approximately 20% in the second postpartum week,22 returning to baseline within the first postpartum month.17,23
40% to 60% above normal, nongravid levels, whereas the increase in RPF at least during the first trimester surpasses that of GFR with an increase in the order of 50% to as high as 80% in one study.11,12,13,14,15,16,17,18,19 Thus, the filtration fraction is thought to fall in a pattern consistent with renal vasodilation. These high levels are maintained through gestational week 36, after which there is a decrease in GFR and a more pronounced decrease in RPF returning the filtration fraction to normal or a slightly elevated value compared to values observed in nonpregnant populations.17,20,21 Despite normalization of RPF, the GFR remains elevated by approximately 41% on the first postpartum day20 and by approximately 20% in the second postpartum week,22 returning to baseline within the first postpartum month.17,23
The relationship between GFR and its determinants can be assessed using the following equation:
where ΔP is the transcapillary hydraulic pressure difference or the pressure generated across the glomerulus; πGC is the mean glomerular intracapillary oncotic pressure, the force that opposes the formation of glomerular filtration; and Kf is the glomerular ultrafiltration coefficient—that is, the product of the surface area available for filtration and the hydraulic permeability (k), which is the permeability to ultrafiltrate across the three layers of the glomerulus. As not all of these individual determinants can be directly measured in humans, and are instead inferred from complex mathematical modeling and theoretical analysis, the mechanisms of hyperfiltration during human pregnancy are not fully understood.24
The hyperfiltration that accompanies human pregnancy, however, appears to result primarily from depression of the oncotic pressure (πGC) in the plasma that flows axially along the glomerular capillaries. The reduction of πGC in pregnancy is attributable to two phenomena. The first is a hypervolemia-induced hemodilution that lowers the protein concentration and oncotic pressure of plasma entering the glomerular microcirculation.25,26,27,28 The second is the elevated rate of RPF.11,12,15,16 Hyperperfusion of glomeruli blunts the extent to which the axial protein concentration and oncotic pressure can increase along the glomerular capillaries during filtrate formation.29
Alternative explanations for increased GFR might include an increase in either Kf or ΔP. Utilizing a mathematical modeling of neutral dextran sieving coefficients to examine the determinants of the GFR in 13 healthy women late in pregnancy, investigators confirmed hyperfiltration was accomplished by an increase in RPF, but suggested perhaps a slight increase in Kf without any alteration in ΔP.30 Our study that assessed determinants of GFR in the early postpartum period could not, with certainty, exclude a role for increased ΔP as an increment in either ΔP of 16% or an approximate increase in Kf of 50% would be necessary to account for the increased GFR in the postpartum period given the normalization of RPF and hence πGC.22 However, one must underscore the theoretical nature of these approaches in pregnant women, as direct micropuncture studies of the determinants of ultrafiltration are obviously not possible in humans. In rodent models, micropuncture studies consistently demonstrate a marked and parallel decrease in afferent and efferent vascular resistance resulting in an increased RPF without intraglomerular hypertension (increased ΔP).31,32,33 Even following repeated pregnancies34 or after a five-sixths reduction in renal mass35 (an extreme example of compromised kidney function), the mechanism of renal accommodation to gestation did not change and there was no potential explanation for the pregnancy-associated renal damage often noted in young women with kidney disease. Further, no adequate human physiologic studies have been performed in diseased states and studies in humans that have utilized protein loading to assess potential renal reserve for accommodation have proven equivocal.36,37 Thus, one cannot state with absolute certainty that the accommodation to pregnancy in a woman with advanced underlying renal disease does not involve an increase in intraglomerular pressure (ΔP) that could potentially have a long-term damaging effect on kidney function, and this is an area of renal physiology worthy of future study.
Tubular Function
Although precise mechanisms are not completely understood, the enhanced GFR that accompanies healthy pregnancy along with altered tubular reabsorption may be responsible for increased urinary levels of glucose, amino acids, uric acid, and protein. This classic teaching, however, is based on sparse data that in many scenarios lacks obvious clinical relevance.
In the nonpregnant state, healthy kidneys efficiently reabsorb glucose (>90%) and glycosuria is a clinical indicator of a filtered load that exceeds the maximal tubular reabsorption capacity (Tm). Despite the increased GFR that accompanies pregnancy, studies that utilized a continuous intravenous glucose challenge with inulin clearance techniques did not document a difference in GFR between women who displayed glycosuria and those who did not, suggesting instead that Tm was significantly decreased in pregnant women who displayed glycosuria.38,39,40 The endogenous mechanism responsible is unclear, but the increased cortisol levels that accompany pregnancy have been postulated as a potential etiology based on observations in nonpregnant diabetic patients.41
The precise incidence of glycosuria in pregnancy is unclear with extensive variability noted between women and even in the same woman at different times during pregnancy.40,42 During an oral glucose tolerance test, 26.9% of 104 patients in the second trimester and 42.8% of 205 patients in the third trimester developed glycosuria,43 but a subsequent retrospective chart assessment of 17,647 pregnancies with normal carbohydrate screening noted an incidence of only 1.6% on routine clinical screening.44 Further, no relationship of glycosuria to clinical diabetes has
been demonstrated, as the majority of women who demonstrate glycosuria have normal glucose tolerance, and even obviously diabetic patients do not consistently demonstrate glycosuria. A theoretical risk of the altered proximal tubular glucose reabsorption might apply to pregnant diabetic patients demonstrating increased susceptibility to hypoglycemia,45 but this has never been proven.
been demonstrated, as the majority of women who demonstrate glycosuria have normal glucose tolerance, and even obviously diabetic patients do not consistently demonstrate glycosuria. A theoretical risk of the altered proximal tubular glucose reabsorption might apply to pregnant diabetic patients demonstrating increased susceptibility to hypoglycemia,45 but this has never been proven.
A similarly confusing pattern has emerged for increased urinary excretion of amino acids and water soluble vitamins.42,46 The few studies designed to determine mechanisms were inconclusive and noted patterns of excretion were not related to the biologic function or chemical structure of the compound.46 However, it is likely that alterations in both GFR and tubular reabsorption would be needed to account for the magnitude of the some of the excretion rates noted.
Serum uric acid has been documented to be decreased in the first trimester, reach a nadir in the second trimester, and then gradually increase as pregnancy progresses and high renal clearance is necessary to clear the increased production that accompanies fetal and/or placental growth.47,48 Uric acid has been noted to be elevated in pregnancies complicated by preeclampsia,49 and even first trimester uric acid levels have been shown to be elevated prior to the diagnosis of preeclampsia.50,51 In one study, uric acid levels in the highest quartile (>3.56 mg per dL) compared to the lowest three quartiles were associated with an increased risk of developing preeclampsia (aOR [adjusted odds ratio] 1.82; 95% CI [confidence interval], 1.03-3.21), but not gestational hypertension.51 Thus, it is not clear if the noted increase in serum uric acid during pregnancies complicated by preeclampsia is solely due to decreased renal clearance secondary to glomerular endotheliosis or increased production caused by trophoblast breakdown. Cytokine release and ischemia might also contribute to increased serum levels.
In healthy adults, proteinuria is typically defined as a protein excretion rate two standard deviations above the mean or greater than 150 mg per day. Due to the aforementioned physiologic changes that accompany the gravid state, the upper limit for proteinuria in pregnancy has been increased and most obstetric guidelines define significant protein excretion as ≥300 mg in a 24-hour period.52 To date, there have been limited efforts to carefully assess serial urine protein or albumin excretion. One of the largest studies wherein the primary attempt was to establish a range for proteinuria in normal pregnancy included 270 healthy women and noted a mean 24-hour urine protein excretion of 116.9 mg with a 95% upper confidence limit of 259.4 mg.53 These levels corresponded to an albumin excretion rate of 11.8 mg with a 95% upper confidence limit of 28.7 mg with no participants exceeding 30 mg per L. Further, the increase in proteinuria did not mirror the increase in filtration, as it typically increased after 20 weeks’ gestation. This later increase in urine protein was also noted in a study that followed protein-to-creatinine ratios in healthy singleton and twin pregnancies, noting a rise in the mean ratio between 34 to 38 weeks that was more pronounced in the twin pregnancies albeit still not impressively elevated (150 and 220 mg per g creatinine in the singleton and twin pregnancies, respectively).54
Of interest, other studies assessing only urine albumin suggest, in fact, no glomerular leak in the vast majority of healthy pregnancies.55,56,57 In a study that assayed 193 consecutive uncomplicated pregnancies, an upward trend in the albumin-to-creatinine ratio was noted as the pregnancy progressed, but only six women had a ratio in excess of 15 mg per g creatinine.55 A similar study that assayed 95 healthy pregnant women between 16 and 20 weeks’ gestation demonstrated only four women with an albumin/creatinine (A/C) ratio greater than 17 mg per g creatinine, of which two proceeded to develop preeclampsia.56 Another study confirmed the slightly higher levels of urine albumin in late pregnancy that further increased in labor, but again noted that the vast majority of the values did not exceed the upper limit of normoalbuminuria.57 The presence of an alternative proteinaceous material is also possible, as one study did demonstrate the protein-to-creatinine ratio exceeded the albumin-to-creatinine ratio more than might be expected.49 In summary, data establishing this well-subscribed higher upper limit for normal proteinuria in pregnancy is suspect at best by the sheer paucity of large, carefully conducted studies with serial measurements, and the presence of significant proteinuria cannot simply be ascribed to the hyperfiltration that accompanies the gravid state.
Electrolytes and Acid-Base Balance
An intricate balance of natriuretic and antinatriuretic factors governs gestational changes in electrolytes (Table 59.1). During pregnancy, total body sodium levels increase significantly by an average of 3 to 4 mEq per day to ultimately peak at an increase of approximately 900 to 1,000 mEq.58 Elevations in GFR cause an increase in sodium filtration from 20,000 to 30,000 mEq per day, whereas increments in progesterone59 and atrial natriuretic peptide (ANP)60,61 levels blunt tubular reabsorption. Other factors that may promote natriuresis include decrements in serum albumin concentration and increments in prostaglandins and melanocyte stimulating hormone.62 These changes are counteracted by the antinatriuretic effect of aldosterone and deoxycorticosterone, which increase drastically in the third trimester of pregnancy. Of interest, aldosterone is particularly responsive to volume levels, and volume expansion with saline has been shown to suppress aldosterone.63 In contrast, deoxycorticosterone is produced through extra-adrenal hydroxylation of progesterone64 and is not suppressible with dexamethasone.65 Therefore, aldosterone may play a more important role in sodium homeostasis whereas deoxycorticosterone might represent an important mechanism to attenuate the natriuretic effects of progesterone. Glomerulotubular changes may also facilitate sodium resorption through increased reabsorption in the proximal and distal tubules.66 Some authors have suggested that this may be mediated by
increments in number of renal Na+/K+ ATPase,67 but this is controversial.68 Overall, the interplay of natriuretic and antinatriuretic factors is complex and varies during different periods of gestation.
increments in number of renal Na+/K+ ATPase,67 but this is controversial.68 Overall, the interplay of natriuretic and antinatriuretic factors is complex and varies during different periods of gestation.
TABLE 59.1 Major Physiologic Changes Associated with Sodium and Potassium Balance in Healthy Human Pregnancy | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Given the mineralocorticoid-induced retention of sodium, it is surprising that total body potassium increases by up to 320 mEq,58 while serum potassium decreases.47,69 Potassium balance was preserved in studies involving mineralocorticoid administration to gravidas,70 while a similar maneuver in males produced decrements in potassium suggesting pregnancy may be responsible for attenuating the kaliuretic effects of mineralocorticoids.70 These authors went on to identify progesterone as the key factor in facilitating potassium retention.70 Pregnancy-induced alterations in potassium handling have important ramifications for the management of diseases associated with potassium processing. For instance, increments in potassium that are noted in sickle-cell anemia may be amplified in pregnancy.71 Of interest, this amplification may not be secondary to potassium retention, but instead due to alterations in aldosterone release. It has also been hypothesized that the net potassium retention in pregnancy may attenuate the hypokalemia induced by diseases such as Bartter syndrome.72 However, exacerbation of Bartter syndrome is more likely given the decrements in serum potassium that characterize normal pregnancy. In fact, complicated pregnancies with an increased need for potassium supplementation have been consistently reported in women with Bartter syndrome.73,74 Altogether, it is imperative that health care providers consider pregnancy-induced alterations in electrolytes during clinical decision making. A recent report by Larsson et al. that details biochemical reference values in normal pregnancy can facilitate this process.75
Acid-base balance is also altered during pregnancy.76 Respiratory alkalosis is induced by an increase in tidal volume77,78 and a concomitant decrease in arterial partial pressure of carbon dioxide (PaCO2).79 A compensatory decrease in plasma bicarbonate is noted along with decrements in hydrogen ion levels.79 Pregnancy-induced elevations in estrogen and progesterone have been implicated as the key factors in elevating minute ventilation, and therefore initiating these downstream changes.80
Blood Pressure and Volume Status in Pregnancy
In parallel with these absolute elevations in electrolytes, body water increases by approximately 8 L81 and plasma volume increases by 1.2 L,82 whereas plasma osmolality falls.47,60,69,82 Such physiologic adjustments are in stark contrast to the nonpregnant state where sustained hypervolemia and decrements in plasma osmolality would result in high blood pressure and remarkable diuresis. Instead, blood pressure begins to decrease early in the first trimester and reaches a nadir between 18 and 24 weeks of gestation11,83 due to significant systemic vasodilatation likely mediated through an altered balance of an array of vasodilatory and vasoconstricting hormones including, but not limited to, nitric oxide and endothelin, prostacyclin and prostaglandin, relaxin, as well as insensitivity to components of the renin angiotensin system (RAS).
The events initiating these changes are not completely understood, but human chorionic gonadotropin (HCG)-induced increased production of relaxin by the corpus luteum may facilitate vasodilation in normal pregnancy.84 In animal models, relaxin upregulates vascular gelatinase
activity, thereby contributing to vasodilation and reduced myogenic reactivity of small arteries through activation of the endothelial endothelin B receptor-nitric oxide (NO) pathway.84 A recent study noted a similar effect in small subcutaneous human arteries incubated with relaxin that was shown to be mediated through vascular endothelial growth factor (VEGF).85 Thus, angiogenic factors may also have an important function in the increased production of NO and prostacyclin in pregnancy via pathways involving phospholipase C (PLC), mitogen-activated protein kinase (MAPK), and protein kinase C (PKC).86 The importance of NO-mediated vasodilatation in the normal vascular adaptation to pregnancy has also been demonstrated in studies that utilize flow-mediated vasodilatation (FMD). In healthy pregnant women, an endothelial NO synthase Glu298Asp polymorphism was noted to be associated with differences in endothelium-dependent dilation at 12 weeks’ gestation87 and the concentration of l-homoarginine, another substrate for NO, has been shown to positively correlate with FMD (r = 0.362, P = 0.006).88
activity, thereby contributing to vasodilation and reduced myogenic reactivity of small arteries through activation of the endothelial endothelin B receptor-nitric oxide (NO) pathway.84 A recent study noted a similar effect in small subcutaneous human arteries incubated with relaxin that was shown to be mediated through vascular endothelial growth factor (VEGF).85 Thus, angiogenic factors may also have an important function in the increased production of NO and prostacyclin in pregnancy via pathways involving phospholipase C (PLC), mitogen-activated protein kinase (MAPK), and protein kinase C (PKC).86 The importance of NO-mediated vasodilatation in the normal vascular adaptation to pregnancy has also been demonstrated in studies that utilize flow-mediated vasodilatation (FMD). In healthy pregnant women, an endothelial NO synthase Glu298Asp polymorphism was noted to be associated with differences in endothelium-dependent dilation at 12 weeks’ gestation87 and the concentration of l-homoarginine, another substrate for NO, has been shown to positively correlate with FMD (r = 0.362, P = 0.006).88
With respect to the RAS, it is the lack of vascular response that is integral for achieving decrements in blood pressure during pregnancy. In normal pregnancy, components of the RAS are upregulated. Prorenin, released from the ovaries, parallels the increase of β-HCG peaking at 10 times the usual blood level.89 Angiotensinogen also increases gradually throughout pregnancy in response to increasing estrogen levels, as does plasma renin activity, possibly in response to progesterone. Increased plasma renin activity results in increased levels of ANG II,90,91 but, as in the luteal phase of the normal menstrual cycle, resistance to its pressor effects characterizes normal pregnancy.92 Vascular insensitivity to ANG II infusion has been demonstrated in healthy pregnant women93,94 and reduced sensitivity of the renal circulation has been demonstrated in pregnant rats.95 Although the mechanism of resistance to the effects of ANG II remains elusive, increased plasma levels and urinary excretion rates of ANG1,2,3,4,5,6,7 have been documented in human pregnancy.90,96 The increased ANG1,2,3,4,5,6,7-to-ANG II ratio may be critical for maintaining the decreased blood pressure that is characteristic of healthy human pregnancy.
Maintenance of hypervolemia is another important deviation from the nonpregnant state. The initial shift toward volume retention is dependent on the lowering of the osmotic threshold for AVP release thereby allowing for continued secretion of the hormone.97 In mid to late pregnancy, AVP levels increase97 in order to compensate for increments in vasopressinase-mediated clearance of the hormone.98 Altogether the preservation of equilibrium is not a static process. Instead, there is a complex and dynamic interplay of increments and decrements in hormone control systems. It is, therefore, not surprising that three major hypotheses have emerged to explain the hypervolemia that attends the gravid state.99 The “underfill” hypothesis is founded on the drastic depression in systematic vascular tone that is noted in early gestation.60 This reduction in pressure results in a transient state of hypovolemia or underfill and the induction of compensatory hormones such as the RAS and vasopressin. Accordingly, there is a subsequent shift toward increased thirst and volume retention. In addition, investigators suggest that the threshold for AVP secretion is also lowered in an attempt to correct underfill. In contrast, the “normal fill” hypothesis suggests that hypervolemia is recognized as the new hemodynamic set point in pregnancy. Increments in β-HCG have been implicated as an initial and independent trigger for reductions in the AVP threshold,100,101 and water intake and retention would increase until plasma osmolality decreases in parallel. Lastly, the “overfill” hypothesis stipulates that the primary change in pregnancy is fluid retention as opposed to decreased vascular tone and intravascular expansion.99,102 The aforementioned elevations in natriuretic factors would, therefore, be consistent with an overfilled state.99 Of interest, some authors have suggested that each of the three hypotheses are correct and all occur in gestation,99 but the temporality of these events has not been clearly established.
A particularly important aberration in volume homeostasis during pregnancy is diabetes insipidus (DI). DI frequently occurs in the third trimester of pregnancy and is characterized by polydipsia and dilute polyuria. At a clinical level, DI during gestation can present as a resurgence of preexisting central or nephrogenic DI, transient DI of pregnancy, or a sequelae of Sheehan syndrome. Given the pregnancy-induced increments in vasopressinase, it is conceivable that AVP levels will further decrease and DI would be exacerbated by pregnancy. In fact, the resurgence of latent DI is documented in the literature.103 Similarly, DI may be subclinical prior to gestation, but become symptomatic during pregnancy. As the increase in vasopressinase correlates with the timing of increments in trophoblastic mass,98 it has been also speculated that women with multigestational pregnancies may have higher vasopressinase levels and therefore be at increased risk for DI.104,105,106 However, this hypothesis has not been directly examined in the literature.
Accumulation or increased activity of vasopressinase may also underlie the development of a transient form of DI during pregnancy.107 Although vasopressinase normally undergoes hepatic clearance during pregnancy, impairments in liver function increase circulating levels of the enzyme thereby leading to decrements in AVP, ultimately resulting in DI.103 Of interest, the hepatic dysfunction noted in HELLP syndrome,106,108 and acute fatty liver of pregnancy109 may be associated with transient DI of pregnancy. Therefore, the diagnosis of DI should also prompt increased vigilance for preeclampsia and hepatic dysfunction. Current treatment for resurgence of latent DI, or transient DI of pregnancy, involves administration of desmopressin (DDAVP), a vasopressinase resistant analogue of AVP. DDAVP can be safely used in pregnancy110 and its transfer to breast milk is limited.
Lastly, an uncommon, but important cause of DI is Sheehan syndrome, characterized by postpartum hemorrhage leading to avascular necrosis of the pituitary gland. It can
also produce diabetes insipidus if the posterior pituitary is affected.111,112,113 However, the independent blood supply of the anterior and posterior pituitary may be protective.112 Nevertheless, polyuria and polydipsia postpartum following significant hemorrhage should raise the appropriate suspicion.112
also produce diabetes insipidus if the posterior pituitary is affected.111,112,113 However, the independent blood supply of the anterior and posterior pituitary may be protective.112 Nevertheless, polyuria and polydipsia postpartum following significant hemorrhage should raise the appropriate suspicion.112
MONITORING RENAL FUNCTION DURING PREGNANCY
Glomerular Filtration Rate
The gold standard for determining GFR in pregnancy remains a carefully timed clearance utilizing either inulin or iothalamate plasma disappearance techniques or carefully timed urine clearances through a Foley catheter, but even in research settings these methodologies have become scarce. Further, clearance methodology, including a timed creatinine clearance, is hampered by the dilated urinary system and the potential bladder retention that accompanies the gravid state, resulting in an underestimation of the true GFR even when creatinine clearance is measured.114 GFR equations, including the Cockcroft-Gault and the MDRD, can substantially overestimate or underestimate GFR and cannot be recommended for use in clinical practice.114,115,116 A recent study that utilized Bland and Altman methodology noted the Cockcroft-Gault equation to underestimate GFR by 25% in 23% of the cases studied and to overestimate GFR by 25% in 16% of the cases studied.114 The MDRD equation, on the other hand, underestimated GFR by 25% in 61% of the cases studied without any cases wherein there was a significant overestimation.114 The newer CKD-EPI equation, which has been deemed superior to the MDRD when assessing patients with higher rates of GFR,117 remains to be assessed in pregnancy.
Thus, trends in the serum creatinine are typically used to assess for renal insufficiency in pregnant women, but the inverse hyperbolic relationship between serum creatinine and GFR is blunted in the elevated range of the latter that is typically associated with pregnancy. A comparison of the serum creatinine and GFR as measured by inulin clearance revealed that the often profound depression in Kf that accompanies preeclampsia could not be appreciated by evaluation of the serum creatinine.118 Although statistically significantly different, the serum creatinine remained in the normal range for both groups with a value of 0.85 ± 0.22 mg per dL in preeclamptic patients and 0.60 ± 0.10 mg per dL in healthy gravid controls, despite a loss of GFR in excess of 50%.
Cystatin C is a potential assay to detect subtle changes in GFR during pregnancy. However, to date, no serial longitudinal studies, spanning all three trimesters and utilizing a gold standard technique for GFR measurement, have been done to determine the value of cystatin C to reflect early changes in GFR. Further, there is evidence to suggest a placental source also exists and cystatin C may be released in response to placental ischemia. Cysteine-proteases are felt to be important for trophoblast invasion and are controlled by inhibitors such as cystatin C. Increases in placental expression of cystatin C at the mRNA and protein level have been noted in women with preeclampsia compared to women with normal pregnancies suggesting that an increase in placental production of cystatin C may contribute to the higher maternal levels seen in women with preeclampsia.119 Although the process is not fully understood, an increase in the synthesis and secretion of cystatin C may be associated with poor placentation, consequently complicating interpretation of cystatin C as a marker of GFR.
Proteinuria
As in the nonpregnant population, issues exist with all the methods used to quantify urine protein. The accuracy of the urine dipstick for predicting meaningful proteinuria is poor with numerous false-negative and false-positive results due to either dilute or concentrated samples, respectively. A systematic review identified only six studies of adequate methodologic quality producing a pooled positive likelihood ratio of 3.48 (95% CI 1.66-7.27) and a negative likelihood ratio of 0.6 (CI 0.45-0.8) for predicting 300 mg per day of urine protein at the 1 + or greater threshold on urinalysis.120 Despite this lack of accuracy, urinalysis as an initial screen is still frequently utilized by the obstetrical community to assess for abnormal proteinuria.
To date, a multitude of studies have correlated either the protein-to-creatinine or the albumin-to-creatinine ratio to the 24-hour urine collection or shorter timed collections typically demonstrating highly positive correlations as might be expected121,122,123 and larger systematic reviews do confirm the ability of ratios to identify clinically meaningful urine protein,124 irrespective of the time of collection,125 making it a tool that can be utilized in the outpatient clinic setting. Correlation, however, would not be the optimal assessment tool to compare two obviously related measures. To date, only a single study utilized the appropriate Bland Altman test to confirm agreement between the 24-hour urine protein and the protein-to-creatinine ratio.126 These authors confirmed the correlation between the two measures and examination of the plots suggests (like the systematic reviews) that at lower levels of urine protein, there is a high level of agreement, but the ratio is less precise at higher levels of urine protein not unlike the nonpregnant state.
Thus, a carefully timed urine protein collection along with an assessment of creatinine excretion to ensure adequacy of the collection remains the most commonly used test for the quantification of urine protein in pregnancy. The potential for inadequate collection due to significant dilatation of the urinary tract system, however, is an issue that remains to be adequately addressed and clarified. One study noted a high error rate (13%-54%) when the adequacy of the collection was assessed based on the predicted creatinine excretion for prepregnancy maternal weight.127 In a recent excellent review on the topic, the author recommended adequate hydration and maintaining the lateral recumbent
position for an hour prior to initiating and completing the collection to reduce the potential errors from retention.128 Although rarely practiced clinically, such techniques should at least be utilized in future studies wherein the careful assessment of proteinuria is required.
position for an hour prior to initiating and completing the collection to reduce the potential errors from retention.128 Although rarely practiced clinically, such techniques should at least be utilized in future studies wherein the careful assessment of proteinuria is required.
Kidney Biopsy
As mentioned, significant proteinuria in a pregnant woman should not simply be attributed to the hyperfiltration of pregnancy. If the clinical presentation includes nephrotic syndrome or deterioration in renal function early in pregnancy without an established diagnosis, a kidney biopsy can be done to assist with the diagnosis and guide treatment. Data is limited with respect to the safety of kidney biopsy in pregnant women. An early study noted bleeding complications to be almost three times more common in pregnant women with serious complications arising including a patient death.129 However, this study predated ultrasound guidance and the diagnosis on a number of the biopsies was preeclampsia with significant hypertension that should have precluded the procedure. The only sizable series was published in 1987 reporting a low complication rate of 4.5% based on 111 renal biopsies in 104 women over 20 years.130 A smaller subsequent case series confirmed safety in women <30 weeks’ gestation.131 Most guidelines, therefore, come from expert opinion recommending a cutoff of approximately 32 weeks’ gestation,132 as the further along in gestation, the more likely that preeclampsia may be factoring into the presentation. In the presence of possible preeclampsia, a kidney biopsy should not be applied indiscriminately, given that the safety of the procedure can be further compromised by evolving hypertension, abnormal coagulation indices, and a low hemoglobin.129 On the other hand, the initiation of steroid or immunosuppressive therapy on the speculation of a potential glomerular-based disease is also not without risk.
PREPREGNANCY RISK STRATIFICATION AND OPTIMIZATION
Prognostication of an individual woman’s pregnancy-associated risk in the setting of chronic kidney disease (CKD) remains profoundly challenging. The literature is complicated and incomplete, and therefore divergent opinions arise with respect to the impact of kidney disease on pregnancy outcome as well as the impact of pregnancy on future CKD progression. The many issues including nonhomogeneity in the classification of the maternal condition (renal function, proteinuria, and hypertension), the frequent absence of preconception baseline data, and the many different definitions of relevant pregnancy outcomes—particularly the nearly impossible task of diagnosing preeclampsia superimposed on CKD wherein hypertension and proteinuria are often already present—are beautifully summarized in a recent excellent systematic review of the literature.133 Suffice to say, prepregnancy renal insufficiency, proteinuria, and hypertension all likely factor toward untoward maternal and fetal outcomes in an additive manner.
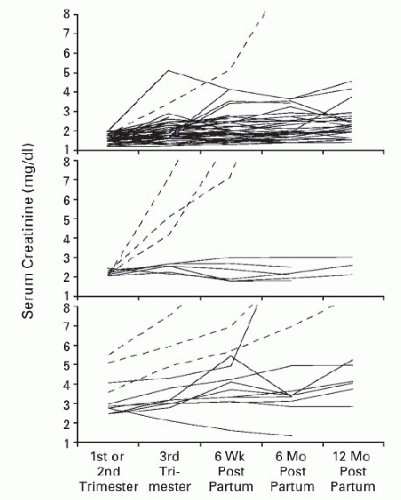
Early studies tended to utilize the serum creatinine to stratify pregnancy risk. A typical stratification schema defined mild renal insufficiency as a serum creatinine less than 123 µmol per L (1.4 mg per dL), moderate renal insufficiency as 124-220 µmol per L (1.4-2.4 mg per dL), and severe renal insufficiency as a serum creatinine exceeding 221 µmol per L (2.5 mg per dL). As the grade of renal insufficiency increased, the healthy accommodation to pregnancy (GFR increase with a simultaneous drop in the serum creatinine) was documented to occur in only approximately 50% of women within the moderate category and in none in the severe renal insufficiency category.134,135 In a classic paper by Jones and Hayslett published over two decades ago, they assessed pregnancy outcome in women with mild, moderate, and severe renal insufficiency as defined above and noted pregnancy-related loss of kidney function in a staggering 43% of pregnancies of which 10% rapidly progressed to end-stage renal disease (ESRD).136 Of interest, not all the accelerated loss occurred only in those with the most severe renal compromise (Fig. 59.3). Both proteinuria and hypertension
are also important factors with respect to the risk for progression that are difficult to examine simultaneously in small single center experiences.135 Further, the serum creatinine is likely too imprecise to be utilized to stratify women prior to pregnancy as it does not take into account patient size and muscle mass, and in young women serum creatinine is often inadequately reflective of the actual degree of histologic renal damage. Tubulointerstitial changes involving in excess of 20% of the cortical area, glomerulosclerosis, and severe arteriolar hyalinosis have all been deemed important with respect to pregnancy outcome.137,138
are also important factors with respect to the risk for progression that are difficult to examine simultaneously in small single center experiences.135 Further, the serum creatinine is likely too imprecise to be utilized to stratify women prior to pregnancy as it does not take into account patient size and muscle mass, and in young women serum creatinine is often inadequately reflective of the actual degree of histologic renal damage. Tubulointerstitial changes involving in excess of 20% of the cortical area, glomerulosclerosis, and severe arteriolar hyalinosis have all been deemed important with respect to pregnancy outcome.137,138
More recently, therefore, studies have prognosticated pregnancy outcome on the basis of a calculated GFR, which has served to increase the reported prevalence of CKD in pregnancy significantly from <1% to 3% as women with more subtle renal insufficiency are identified.133,139 A recent study assessed pregnancy outcome in women with stage 3-5 CKD, excluding women with diabetes and lupus.140 They utilized the MDRD formula to calculate the GFR and stratified women into four groups based on GFR (>40 or ≤40 mL/min/1.73 m2) and the baseline level of proteinuria (<1 or ≥1 g per d). In women with a GFR in excess of 40 mL/min/1.76 m2, there was no change in the rate of progression before and after pregnancy irrespective of the degree of urine protein. In women with a GFR ≤40 mL/min/1.73 m2, the change in the rate of the postpartum decline in renal function was governed by the presence or absence of proteinuria, wherein the rate of progression increased from −0.55 mL/min/month to −1.17 mL/min/month. Gestational age decreased across the four groups as did birth weight. They concluded that only women with advanced kidney dysfunction and proteinuria need be counselled aggressively with respect to pregnancy, but their study overall was small (n = 49) and requires confirmation specifically in the group with GFR >40 mL/min/1.73 m2 and with significant proteinuria as there were only six women in this group. Further, a subsequent study did note adverse outcomes even at early stages of CKD that associated with the degree of proteinuria and hypertension, but was limited by the fact that a first trimester serum creatinine was utilized in quite a number of cases, which could have served to reclassify more severe renal insufficiency to stage I CKD following accommodation to pregnancy.133 The most recent study utilized the CKD-EPI equation to calculate eGFR from a preconception serum creatinine and noted an odds ratio for a composite maternal complication (worsening kidney function or preeclampsia) to be 6.75 (95% CI 1.8-24.8) and an odds ratio for a fetal complication (intrauterine growth restriction [IUGR], preterm birth, and fetal death) to be 2.91 (95% CI 1.19-7.09) when eGFR ranged between 60 and 89 mL/min/1.73 m2.141 A large study that utilized data from the HUNT II cohort, however, noted that the increased risk of preeclampsia in women with an eGFR between 60 and 89 mL/min/1.73 m2 occurred only in women who also had hypertension and that there was a significant interaction between reduced kidney function and hypertension.142 Although interesting, only a fraction of these women had microalbuminuria and therefore this is not the population likely to present to a renal clinic for prepregnancy consultation. Instead, women with identified kidney disease of various histologic types along with varying degrees of renal insufficiency, proteinuria, and hypertension will deserve guidance with respect to preconception planning and existent data suggests caution be exercised in women with established kidney disease. Multicenter efforts with larger numbers of patients will be necessary to refine counselling strategies particularly in the large group of women with moderate disease and to better understand the impact of different types of kidney disease along with current available treatment regimens. Available data for specific disease entities, as well as ESRD, are discussed in later text.
Diabetic Nephropathy
Due to increasing rates of obesity, diabetes mellitus is becoming a growing public health concern. Prepregnancy assessment and optimization can be effective143 and it is, therefore, mandatory to improve pregnancy outcomes. Adequate prepregnancy optimization includes achieving a glycosylated hemoglobin (HbA1c) of ≤7.0%144 and is typically achieved with multiple daily injections of insulin or an insulin pump. In addition to minimizing potential congenital abnormalities,145 meticulous glycemic control improves pregnancy outcomes. In fact, for each 1% increment in HbA1c the adjusted odds ratio for preeclampsia is 1.6 (95% CI 1.3-2.0) whereas it is 0.6 (95% CI 0.5-0.8) for every 1% decrement during the first half of pregnancy.146 The trend to delay childbirth, however, is resulting in more end-organ damage, including diabetic nephropathy, even prior to the consideration of pregnancy, thereby mandating early involvement of nephrology as well as endocrinology in prepregnancy counselling and optimization. The exact manner in which to counsel and optimize a young woman with significant nephropathy, however, is remarkably less clear based on the sparse and often controversial existing literature. Although most series note approximately a 90% live birth rate,147,148,149 risks inherent to a pregnancy complicated by diabetic kidney disease are twofold. Significant fetal risks include poor growth and preterm delivery. Frequent maternal complications include acceleration of hypertension and preeclampsia as well as the potential to hasten progression of underlying nephropathy.
Like other forms of kidney disease, pregnancy outcome is affected by the prepregnancy kidney function, proteinuria, and blood pressure, but the rates of untoward outcomes are likely higher than described in other forms of kidney disease. One study assessing pregnancy outcome categorized women according to their urinary albumin excretion rate.150 Compared to women with normal albumin excretion wherein the rate of preeclampsia was 6%, the rate increased to 42% and 64% in women with microalbuminuria and diabetic nephropathy, respectively. A similar pattern emerged for extreme preterm delivery (<34 weeks’ gestation) wherein the rate was 6% in women with normal urine albumin excretion, 23% in women with microalbuminuria, and finally
45% in women with diabetic nephropathy, defined as urine protein excretion >500 mg daily. However, there were also differences between the groups in both baseline HbA1c and blood pressure. Irrespective, the strong relationship between microalbuminuria and preeclampsia has been noted in other studies,151,152,153,154 including one that noted no additive predictive value of blood pressure151 and in another that adjusted for both baseline hypertension and glycemic control.153 Unfortunately, baseline serum creatinine was rarely reported and never adjusted for as a potential contributor to untoward pregnancy outcome in any of these studies, but has been shown to be an independent predictor of delivery before 32 weeks’ gestation and very low birth weight independent of proteinuria and glycemic control in any trimester.155 Although microalbuminuria and proteinuria are a reflection of established endothelial dysfunction, a well-described correlate of preeclampsia and preterm delivery,156 HbA1c, blood pressure, and baseline kidney function are likely additive with respect to adverse pregnancy outcomes.
45% in women with diabetic nephropathy, defined as urine protein excretion >500 mg daily. However, there were also differences between the groups in both baseline HbA1c and blood pressure. Irrespective, the strong relationship between microalbuminuria and preeclampsia has been noted in other studies,151,152,153,154 including one that noted no additive predictive value of blood pressure151 and in another that adjusted for both baseline hypertension and glycemic control.153 Unfortunately, baseline serum creatinine was rarely reported and never adjusted for as a potential contributor to untoward pregnancy outcome in any of these studies, but has been shown to be an independent predictor of delivery before 32 weeks’ gestation and very low birth weight independent of proteinuria and glycemic control in any trimester.155 Although microalbuminuria and proteinuria are a reflection of established endothelial dysfunction, a well-described correlate of preeclampsia and preterm delivery,156 HbA1c, blood pressure, and baseline kidney function are likely additive with respect to adverse pregnancy outcomes.
In addition to pregnancy-related complications, the potential for progression of nephropathy and accelerated loss of kidney function is serious and deserves careful consideration given the poor outcome of patients with ESRD secondary to diabetes mellitus. Proteinuria has been described to increase throughout pregnancy with nephrotic syndrome (>3 g per day) developing in the vast majority (>70%) who enter pregnancy with diabetic nephropathy (>500 mg per day) and tends to occur along with some increase in blood pressure in the third trimester.157,158,159,160 In those studies that followed urine protein well into the postpartum period significant improvements were noted and in many women the value returned to prepregnancy levels.157,158 The rates of late gestation nephrotic range proteinuria being higher than the quoted rate of preeclampsia in this population reflects the difficulty of diagnosing the syndrome in a population that already has significant proteinuria as well as lack of understanding of the pathophysiologic mechanisms of preeclampsia in this particularly high risk population (see later section, Pathophysiology of Preeclampsia).
Despite the almost uniform worsening of urine protein during pregnancy in women with diabetes mellitus, the literature has been conflicting with respect to pregnancy impact on progression of renal dysfunction. Early studies concluded that pregnancy did not hasten disease progression in diabetes,147,157,158,161,162,163 but these early studies were small with variable follow-up periods and included a spectrum of baseline kidney function with the majority of patients having well preserved kidney function at conception. Those with significant renal insufficiency and nephropathy certainly did deteriorate during pregnancy, but the overall slope toward ESRD, which was already steep, did not change significantly reflecting the overall poor outcome of diabetic nephropathy prior to more widespread use of blockade of the RAS. Studies that more effectively stratified women based on baseline renal function noted that women with well-preserved kidney function at conception had better outcomes whereas moderate to severe renal insufficiency predicted more rapid deterioration in kidney function during and after pregnancy.159,160,164,165 One such study noted a baseline creatinine clearance of 70 mL per min to be a potentially meaningful value with respect to outcome.164 Women who entered pregnancy with a creatinine clearance >70 mL per min were more likely to have an appropriate early pregnancy renal accommodation and stable kidney function after pregnancy whereas women with lower baseline clearance values had a significant decline in renal function at 3 months postpartum (36% lower). Another study that assessed women with more significant baseline renal dysfunction (mean creatinine 1.8 mg per dL) found a significant deterioration in kidney function that was transient in 27% and permanent in 45%.159 It is distinctly possible that superimposed preeclampsia manifesting as worsening proteinuria and kidney function during pregnancy is damaging over the long term. A health administrative study from the Norwegian Renal Registry that identified 2,204 women with pregestational diabetes whose pregnancy was complicated by either preeclampsia, preterm delivery, or a low birth weight baby and noted higher future rates of nephropathy, ESRD, and death.166 Another study noted the median age of children to be 9 (3-17) when the mother expired secondary to complications of renal or cardiac disease,167 reminding us that this is a very vulnerable, high-risk group that is not to be taken lightly.
Despite the great potential for both untoward maternal and fetal outcome, there are precious little data to guide prepregnancy preparation outside of optimization of HbA1c. In one study, for example, hypertension was not treated unless the diastolic blood pressure exceeded 105 mm Hg.157 There is, however, some data to suggest that adequate blood pressure control is important. Although no randomized data exists, one retrospective cohort study compared outcomes in women with a mean arterial pressure either ≥100 mm Hg or <100 mm Hg and noted patients with higher blood pressures were significantly more likely to deliver before 32 weeks’ gestation (38.1% versus 4.6%, P =.007) even after adjusting for duration of diabetes and glycemic control.168 A second prospective study targeted a blood pressure of < 135/85 mm Hg in 117 pregnant women with type 1 diabetes mellitus and noted a trend toward longer gestation and higher birth weight as compared to historical data published prior to theirs from the same geographical region in Europe.169 However, it is difficult to draw comparisons between these small single-centered studies, and the vast majority of the women included in their study did not have even microalbuminuria, a group one might overall expect to do better. Only one group has assessed the potential for prepregnancy optimization in women with significant diabetic nephropathy. They have published two articles advocating for aggressive prepregnancy blockade of the RAS with captopril to lower proteinuria prior to pregnancy along with aggressive glycemic control.170,171 They recommend stopping captopril at the time of conception and effectively demonstrated the ability to lower proteinuria from
a mean value in excess of a gram to a mean value <300 mg. Although proteinuria still increased throughout pregnancy, the rate of increase was not as dramatic as what might be expected from the previous literature and kidney function was stable at 2 years postpartum. Of note, the patients in these two small series had well-preserved kidney function, which likely also factored into favorable long-term outcomes. Irrespective, there are data outside of pregnancy that speak to the potential for prolonged renoprotective effect after cessation of RAS blockade.172
a mean value in excess of a gram to a mean value <300 mg. Although proteinuria still increased throughout pregnancy, the rate of increase was not as dramatic as what might be expected from the previous literature and kidney function was stable at 2 years postpartum. Of note, the patients in these two small series had well-preserved kidney function, which likely also factored into favorable long-term outcomes. Irrespective, there are data outside of pregnancy that speak to the potential for prolonged renoprotective effect after cessation of RAS blockade.172
Of course, anyone prescribing RAS blockade to a woman of child-bearing potential needs to be cognizant of the potential for teratogenicity.173 Second and third trimester teratogenicity secondary to angiotensin-converting enzyme (ACE) inhibition is well described and includes oligohydramnios, neonatal anuria and renal failure, limb contractures, craniofacial abnormalities, pulmonary hypoplasia, and patent ductus arteriosus. Children that survive ACE inhibition/angiotensin receptor blocker (ARB) fetopathy are left with renal insufficiency and profound impairment in the urine concentrating ability likely due to papillary atrophy and disturbed formation of the medullary concentration gradient.174 A more recent publication, however, suggests the potential as well for first trimester teratogenicity.175 This study has a number of issues such as the inclusion of defects not previously described in risk estimates as well as the inability to control for other potential confounders including maternal age, obesity, and diet-controlled diabetes. ARBs may very well be more teratogenic with case reports of significant malformations emerging after first trimester exposure.176,177,178 There are no case reports as yet of teratogenicity after exposure to the newer direct renin inhibitors, but there is no reason to expect they will not be equally or even more teratogenic, and this class of medication also should be used with extreme caution in young women. The risk of teratogenicity must be carefully balanced against the need to prevent progression of diabetic nephropathy. It is, therefore, imperative that physicians educate young diabetic women as to the risks of an unplanned pregnancy as opposed to denying them a potentially renoprotective therapy. Unintentional first trimester exposure does not require termination, but careful fetal imaging is recommended.179 In the postpartum period, there is also no need to deny RAS blockade while breastfeeding. Captopril, enalapril, and quinapril have all been tested and noted to be absent in breast milk and, therefore, can be used if necessary to treat diabetic nephropathy in the early postpartum period.180,181
IgA Nephropathy
Although IgA nephropathy is the most common glomerular-based disease diagnosed in women of childbearing age, there is a remarkable paucity of data in the literature to assist with prepregnancy counselling and management. Most studies, being small and decades old, could not simultaneously assess the impact of kidney function, blood pressure, and proteinuria on pregnancy outcome or potential impact of treatment prior to conception with either immunosuppression or blockade of the RAS.137,182,183,184 Due to the slow insidious nature of the disease, a large proportion of women first come to medical attention during pregnancy without a careful prepregnancy assessment of blood pressure, proteinuria, and kidney function to assist with the understanding of the impact of these variables on the prognostication of pregnancy outcome. The follow-up time used to determine if pregnancy ultimately has an impact on progression is likely inadequate with only 5 years of long-term follow-up in most studies.182,183,185,186,187 Finally, in studies that attempt to assess the impact of histology, older classification systems were used and the baseline clinical correlates of disease were not consistently reported, therefore making this area of literature very difficult to interpret.188,189
Early data would suggest that even mild disease associated with preserved renal function might significantly increase the risks of untoward pregnancy outcomes and worsening maternal condition. In a large analysis of 116 pregnancies, the fetal loss was noted to be 22%.190 Maternal renal function declined transiently in 26%, and was progressive and irreversible in 2%. Proteinuria and hypertension increased in 52% and 62% of women, respectively, and did not resolve after delivery in 13% and 10%, respectively. This study, however, was retrospective with the vast majority of women formally diagnosed by biopsy either during or after pregnancy limiting the collection of careful prepregnancy data.
Also supporting the notion that even mild disease may have consequences in pregnancy are studies that assessed women with isolated hematuria, a marker perhaps of even milder glomerular disease. A study that assessed 276 women, of which 44 had isolated hematuria on their first prenatal visit, noted significantly increased rates of preeclampsia (OR 9.1, 95% CI 2.5-33.7).191 That odds ratio was mitigated albeit still statistically significant in a larger dataset wherein the same authors utilized data from the trial of Calcium for Preeclampsia Prevention (CPEP) noting idiopathic hematuria in 132/4307 (3%) of participants. An almost twofold increased risk was observed for the development of preeclampsia after adjustment for blood pressure, race/ethnicity, and medical center (aOR = 1.89; 95% CI 1.12-3.18).192 However, the most recent and largest study to date (n = 1,000) noted that dipstick positive hematuria was common (20%) and typically did not signify any meaningful renal disease, as 60% of these patients were carefully assessed in the nephrology clinic.193 In this more comprehensive study, microscopic hematuria did not increase the likelihood of preeclampsia, gestational hypertension, or delivery of a small for gestational age baby. Further, a sizable study that assessed women with known thin basement membrane disease, another potential explanation for hematuria, also failed to note rates of pregnancy-related complications that differed significantly from those of the general population.194
The absence of prepregnancy baseline data and the use of serum creatinine to determine baseline renal function likely hampered the interpretation of pregnancy risk in
many of the early studies. In studies wherein the creatinine clearance was calculated, a better understanding of pregnancy risks emerged. Women with preserved kidney function as defined by a creatinine clearance over 70 mL per min were demonstrated to have reasonable pregnancy outcomes.137,186,195 Exceptions were noted in women with difficult to control prepregnancy hypertension (blood pressure [BP] > 140/90 mm Hg)66,69,77 or worsening of hypertension early in pregnancy183,196 as well as in women with significant renal scarring on biopsy that perhaps was not appropriately reflected by clinical measures of renal function. Specifically, worse pregnancy outcomes have been noted in women with sclerosed glomeruli,188,189,195 tubulointerstitial damage that involves in excess of 20% of the cortical area,137



many of the early studies. In studies wherein the creatinine clearance was calculated, a better understanding of pregnancy risks emerged. Women with preserved kidney function as defined by a creatinine clearance over 70 mL per min were demonstrated to have reasonable pregnancy outcomes.137,186,195 Exceptions were noted in women with difficult to control prepregnancy hypertension (blood pressure [BP] > 140/90 mm Hg)66,69,77 or worsening of hypertension early in pregnancy183,196 as well as in women with significant renal scarring on biopsy that perhaps was not appropriately reflected by clinical measures of renal function. Specifically, worse pregnancy outcomes have been noted in women with sclerosed glomeruli,188,189,195 tubulointerstitial damage that involves in excess of 20% of the cortical area,137
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree