Several organizations around the world have developed clinical practice guidelines to define the target level of small-solute clearances required to optimize the health of patients undergoing PD. As would be expected, these guidelines have evolved with our understanding (as discussed previously), particularly with the availability of the results of two large randomized controlled clinical trials.
37,
38 The updated guidelines by organizations in United States, Canada, and Europe are summarized in
Table 83.4.
58,
59,
60,
61,
62 When compared to guidelines published earlier, the current recommendations differ in several important respects. First, most of the guidelines recommend only one measure of adequacy to define the minimum dose of dialysis (Kt/V
urea). Early studies, including the CANUSA study, suggested that patient outcome was more dependent upon total (renal + peritoneal) creatinine clearances rather than total urea clearances.
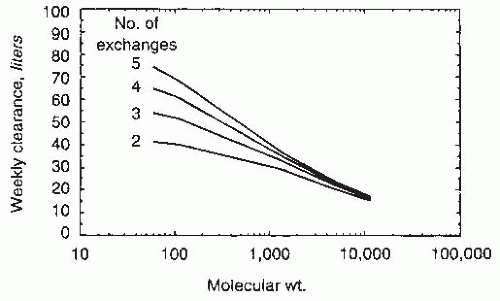
63 However, the contribution of renal creatinine clearance to total (renal + peritoneal) creatinine clearances is substantially greater than of native renal urea clearances. Because creatinine is secreted and urea is reabsorbed by renal tubules, renal creatinine clearance is always higher than renal urea clearance; on the other hand, peritoneal clearances are dependent on the molecular weight of the solute in question. Thus, creatinine clearance (molecular weight, 113) is always lower than peritoneal urea clearance (molecular weight, 60). Consequently, the expected weekly creatinine clearance is different in a patient who is just starting PD with a residual renal Kt/V
urea of 2.0 per week than in an anuric patient with a peritoneal Kt/V
urea of 2.0 per week. Thus, although both markers of solute clearance may be predictors of outcome, the target or goal for creatinine clearance may have to change over time as residual renal function decreases and is replaced by peritoneal clearance.
On the other hand, it appears from outcome studies that the Kt/V
urea target may not need to change. Furthermore, it is now recognized that the stronger relationship of creatinine clearances to patient outcome was a result of the effect of the confounding effect of residual renal function. There is no evidence that peritoneal creatinine clearances are superior in predicting outcome, when compared to peritoneal urea clearance. In light of these considerations, the various expert groups recommend the use of Kt/V
urea alone to determine the dose of dialysis (
Table 83.4). Second, the targets for Kt/V
urea have been changed, such that Kt/V
urea of 1.7 at all times is now considered to be the minimum dose necessary needed for patient well-being. Based on the results of the two recent randomized controlled trials, it is also recognized that some patients may require a higher dose of dialysis to manage uremic symptoms or to achieve euvolemia.
36,
37 Third, except in the CARI guidelines, there are no differences in the definition of minimum dose of dialysis based upon the patients’ transport type (see below). Fourth, some expert groups (Europe and Australia) have defined the adequacy of dialysis based only on peritoneal clearances, whereas others (Canada and the United States) define it based upon total clearances. Fifth, the targets are the same, irrespective of PD modality (CAPD or APD). Finally, volume control is recognized as an additional dimension to define adequate dialysis (see below).