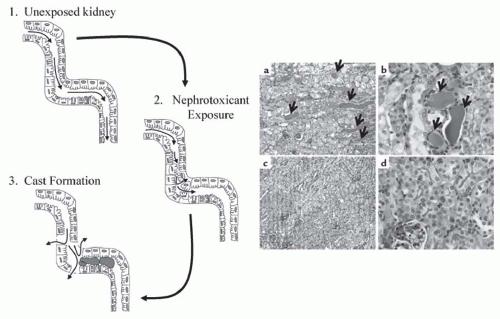
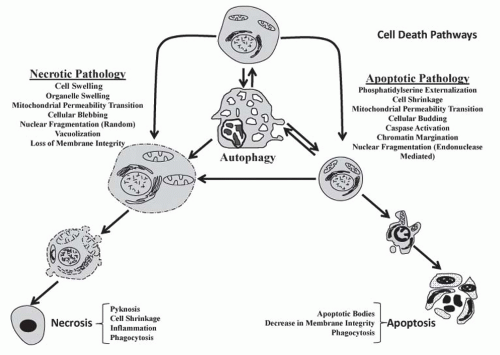
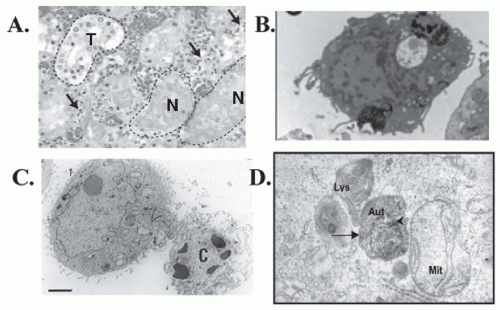
A key difference between necrosis and apoptosis is the activation of caspases in the latter. Caspases are cysteinyl aspartate-specific proteases that belong to an 18-member family.
57,
58,
59 Caspases can be divided into three groups based on structural differences and substrate preferences: initiator caspases (caspase -2, -8, -9, -10, and possibly -12), executioner caspases (caspases -3, -6, and -7), and cytokine processors (caspases -1, -4, -5, -13, and -14). Caspases-15 to 18 have been identified in numerous mammals, but not in humans, with the exception of caspase 16.
59Caspases have numerous other substrates other than DNAases. Initiator caspase substrates include other caspases, the pro-apoptotic protein Bid, α-tubulin, and vinculin.
58 Cytokine caspase substrates include inflammatory mediators such as such 11-18, Pro-IL-1B, and 11-17, whereas executioner caspase substrates include protein kinase C (PKC), focal adhesion kinases (FAK), and the cell cycle regulator p21.
58 Cleavage of these proteins is believed to inhibit futile repair attempts, facilitate apoptosis signaling cascades, and allow for cytoskeleton reorganization and packaging of cell constituents into apoptotic bodies.
80Caspase-3, perhaps the best studied executioner caspase, can also cleave receptors, such as type 1 inositol(1,4,5) P4 receptor, Ca
2+-ATPase, the Na
+/Ca
+ exchanger, and the Na
+/K
+-ATPase pump.
55 The Na
+/K
+ ATPase may also be cleaved by initiator caspases, such as caspase-8 and -9.
81 Cleavage of these receptors is believed to alter ion homeostasis and facilitate decreases in intracellular K
+, which further promotes caspase activation. Cleavage of these receptors also leads to cell size alterations, such as cell shrinkage, early during apoptosis after cleavage of Na
+/K
+ ATPase, or cell rupture due to swelling after Ca
2+-ATPase inactivation during late-stage apoptosis/secondary necrosis.
Role of Mitochondria in Apoptosis
The role of mitochondria in cell death cannot be understated, especially for apoptosis. Mitochondria regulate apoptosis by at least two major processes: maintenance of ATP production and release of pro-apoptotic proteins, such as cytochrome c, Bcl-2 family proteins, and DNAases. In addition, mitochondria regulate apoptosis by participating in Ca
2+ signaling cascades and mediating protease activation.
55ATP is considered to be a requirement for both the initiation and execution of apoptosis.
55 It is required for formation of an apoptosome protein complex (see later), which facilitates the activation of caspase-9. It may also be required for transport of pro-apoptotic proteins into the nucleus.
55 ATP may also represent an important switch point between apoptosis or necrosis; depleting ATP below 30% transforms apoptotic liver cell death to necrotic death patterns.
82 In addition, ATP is needed to maintain Na
+/K
+ ATPase pumps on the plasma membrane, and pump inactivation will eventually lead to cellular swelling, pathologic increases in intracellular Ca
2+, and cellular lysis, which is typical of necrosis.
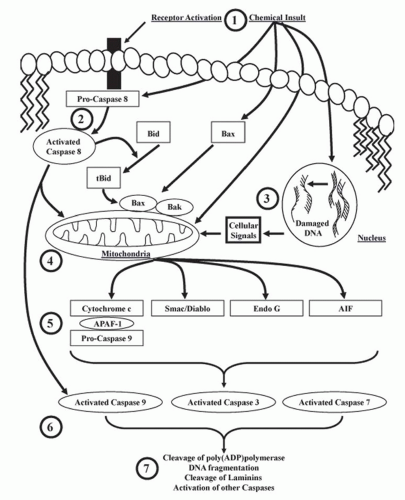
Cytochrome c is a heme protein bound to the inner mitochondrial membrane, transferring electrons between complexes III and IV of the electron transport chain. Release of cytochrome c from mitochondria activates the intrinsic pathway of apoptosis. Cytosolic cytochrome c will bind
to apoptotic protease activating factor 1 (APAF-1), which promotes the binding and proteolytic cleavage of procaspase-9 to caspase-9 (the apoptosome),
83 and then activated caspase-9 cleaves and activates executioner caspases (i.e., caspases-3, -6, and -7) (
Fig. 30.4). Nephrotoxicants known to induce cytochrome c release in correlation with apoptosis include cisplatin and DCVC.
71,
84 Cytochrome c release from the mitochondria is associated with a decrease in the mitochondrial inner membrane potential and the accumulation of several pro-apoptotic proteins such as Bad, Bax, and Bax at the mitochondria (
Fig. 30.4). Other proapoptotic proteins released from the mitochondria include apoptosis-inducing factor (AIF), Smac/Diablo, Omi, and Endo G (
Fig. 30.4).
70,
71,
85,
86,
87,
88,
89,
90,
91,
92Bad, Bak, Bax, and Bid belong to the Bcl-2 family of pro-apoptotic proteins, which are characterized by specific regions of homology, termed Bcl-2 homology domains.
93 Under nonstressed conditions, these proteins exist bound to proteins in the mitochondria and cytosol.
70 After toxicant exposure, Bax, Bid, or Bak can dissociate and translocate to the mitochondria which initiates the formation of a pore complex that causes membrane rupture
55 and subsequent loss of mitochondrial membrane potential, facilitating the release of cytochrome c, Endo G, Smac/Diablo, Omi, and AIF (
Fig. 30.4).
68,
70,
74 Bid mediates apoptosis induced by hypoxia and ATP depletion in cultures of rat RPTC
94; Bax mediates proximal tubular apoptosis in mice treated with cisplatin in vivo
65; and Bak is elevated during apoptosis in primary bovine glomerular endothelial cells induced by TNF-α or lipopolysaccharide (LPS
95) or during ischemiareperfusion-induced renal cell apoptosis in mice.
96In contrast to Bax, Bid, and Bak, Bcl-2 is an anti-apoptotic protein.
60 Increased Bcl-2 prior to toxicant exposure protected numerous cells, including renal cells,
96 from toxicant-induced apoptosis.
96 The protective effect of Bcl-2 may be the result of its ability to bind Bax, Bid, and Bak, preventing them from inducing mitochondrial pore formation, altering mitochondrial membrane permeability, initiating the release of mitochondrial pro-apoptotic proteins, and activating caspases.
97 Overexpression of Bcl-2 protected against ATP-depletion-induced apoptosis in cultures of rat RPTC,
94 and upregulation of Bcl-2 protected kidney epithelial cells both in vitro and in vivo against apoptosis induced by hypoxia, azide, cisplatin, and staurosporine.
98AIF is released from mitochondria in response to decreases in the mitochondrial membrane potential induced by ATP depletion
86,
99; ischemia-reperfusion; anti-fas antibodies
100; or exposure to high concentrations of Ca
2+,
101 t-butyl hydroperoxide,
101 or atractyloside.
101 Cellular pathologies associated with AIF release are similar to those seen with caspases (chromatin condensation and oligonucleosomal DNA fragmentation).
100 Recent studies suggest that increases in cytosolic Ca
2+ and calpain activation also facilitate the release of AIF from mitochondria
102; and studies in LLC-PK1 cells support this hypothesis.
103AIF is a protease with properties similar to caspases, including being inhibited by
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-fmk), a commonly used broad spectrum caspase inhibitor.
86 Thus, the decrease in renal cell death observed in the presence of Z-VAD-fmk may be a result of AIF or caspase inhibition. AIF can induce DNA fragmentation independently of caspases.
69 AIF is released in opossum kidney (OK) cells after ATP depletion-induced by sodium cyanide and 2-deoxy-D-glucose.
87,
99 AIF is also activated in HEK293 cells after exposure to cadmium,
104 in LLC-PK1 cells after exposure to cisplatin,
105 and in OK cells after exposure to the peroxisome proliferator-activated receptor agonist ciglitazone.
106Smac/Diablo is a pro-apoptotic protein released from the mitochondria to the cytosol during apoptosis. It blocks antiapoptotic activity of inhibitors of apoptosis proteins (IAP), which increase apoptosis.
89 The ability of Smac/Diablo to promote apoptosis is not exclusively a result of its ability to bind IAP.
107 Smac/Diablo functions at the same level of executioner caspases, but downstream of the Bcl-2 family of proteins.
90Smac/Diablo is expressed in the mouse kidney and in several renal cell models.
108 It mediates apoptosis in vivo in mice after treatment with high concentrations of folic acid or after exposure of cultures of renal epithelial cells to TNF-α.
89 Increased expression of Smac/Diablo potentiates TNF-α- and etoposide-induced apoptosis in HEK293 cells
107; however, similar to several other pro-apoptotic proteins, expression of Smac/Diablo is not essential for apoptosis in kidney cells. For example, acetaminophen-induced renal cell apoptosis proceeds in a caspase-dependent manner in the absence of Smac/Diablo activity.
109Omi is a mammalian serine protease homologous to bacterial HtrA endoprotease.
110 Omi localizes to the mitochondria and is expressed ubiquitously in a number of cell types including RPTC.
91 Omi is released from the mitochondria after exposure to apoptotic stimuli and binds to, and cleaves, IAP.
91 Omi-directed degradation of IAP facilitates caspase activation and the subsequent biochemical and morphologic features of apoptosis. In addition, Omi can translocate to the nucleus and activate the transcription factor p73, which induces pro-apoptotic proteins such as bax.
69 Omi participates in both caspase-independent and caspase-dependent cell death,
69,
111 an event that has been proven using either siRNA against Omi, or a synthetic inhibitor, called ucf-101, in in vitro and in vivo models, including primary cultures of mouse RPTC.
91,
111 More work is needed to determine if Omi can mediate cell death induced by other nephrotoxicants.