RENAL SODIUM AND WATER RETENTION IN THE NEPHROTIC SYNDROME: CLINICAL OBSERVATIONS
For a long time the theory of transcapillary fluid transport, governed by the principles of the Starling equation, dominated the discussion of edema formation in the nephrotic syndrome. A reduction in the amount of circulating albumin,
which is the major determinant of oncotic pressure, will promote transport of water across the capillary wall toward the interstitium. As a result plasma and blood volume will decrease. It was proposed that in patients with a nephrotic syndrome plasma and blood volume were (partly) maintained by sodium and water retention that increased the extracellular volume.
6 This increased sodium reabsorption was attributed to neurohumoral activation and renal hemodynamic changes as a consequence of the decreased blood and plasma volume.
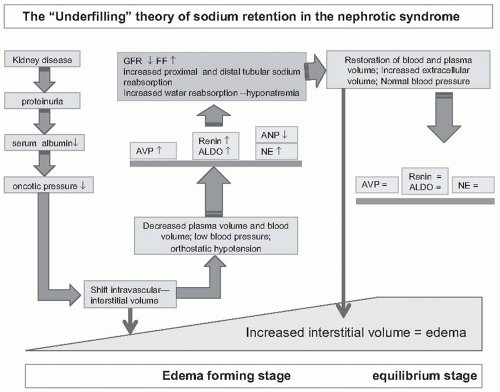
Many studies have provided data that are in line with the sequence of events as depicted in
Figure 69.1, and thus support the underfilling theory of sodium retention in the nephrotic syndrome. There is no doubt that plasma and blood volume can be severely compromised in children with a nephrotic syndrome. Van de Walle et al. reported in detail nine children with multirelapsing nephrotic syndrome due to minimal change disease.
7 These patients were studied during a severe relapse, with serum albumin concentration averaging 1.6 g per dL. The patients had symptoms of hypovolemia such as tachycardia, oliguria, peripheral vasoconstriction, and abdominal pain. These children had low GFR; elevated levels of renin, aldosterone, and vasopressin; and a markedly increased proximal tubular sodium reabsorption. Oliver studied seven children with steroid sensitive nephrotic syndrome, and observed increased urinary norepinephrine excretion in the nephrotic phase.
8 Urinary norepinephrine was positively correlated with plasma aldosterone and negatively with urinary sodium excretion. In studies that followed, these investigators showed that volume expansion with intravenous administration of albumin lowered plasma norepinephrine levels.
9 Gur et al. studied six children with lipoid nephrosis.
10 In the period of nephrosis these patients had reduced electrolyte-free water clearance, compatible with increased proximal tubular sodium reabsorption.
Support for underfilling is not limited to studies in children with steroid sensitive minimal change nephrotic syndrome. Yamauchi and Hopper described 10 adult patients who presented with hypotension and hypovolemic shock as complications of the nephrotic syndrome.
11 These patients had severe hypoalbuminemia, amounting 1.4 g per dL (range 0.4 to 2.2 g per dL). Blood volume was reduced to values ranging from 71% to 92% of the predicted values. Kunagai studied 11 patients with a nephrotic syndrome due to minimal change disease and relatively well preserved renal function.
12 These patients were studied in the stage of edema formation, during diuresis, and in remission. In the edema forming stage, the patients retained sodium and their body weight increased by >0.2 kg per day. Blood pressures were low to normal, ranging from 113/71 to 142/90 mm Hg. In the edema forming stage plasma volume (measured in supine position) was decreased, and plasma renin activity (PRA) and plasma aldosterone concentration (PAC) were increased. PRA correlated with plasma volume and PAC, and sodium excretion was lowest in patients with highest PAC. Evidence to support the role of aldosterone in sodium retention comes from clinical studies, in which spironolactone, a selective mineralocorticoid receptor antagonist, was used. Shapiro et al. studied patients with a nephrotic syndrome and a high sodium intake. Within 3 days after the start of therapy sodium excretion increased from 205 ± 20 mmol per day to 312 ± 13 mmol per day in patients on spironolactone, and remained stable in controls.
13 Other investigators evaluated the role of arginine
vasopressin (AVP). Usberti et al. studied 16 patients with a nephrotic syndrome, all with normal blood pressure and normal renal function.
14 These patients were studied while in equilibrium (no weight gain). For comparison, patients with glomerulonephritis were evaluated. The nephrotic patients had lower plasma sodium concentration and blood volume, and increased levels of plasma AVP, PRA, and urine epinephrine. Patients with the nephrotic syndrome were unable to excrete a water load: maximal urinary flow rate was 4.52 ± 1.71 mL per min (vs. 10.0 ± 2.26 mL per min in controls) and minimal urine osmolality 161 ± 50 mOsm per kg (vs. 83 ± 8 mOsm per kg). The conclusion that in the nephrotic syndrome AVP was non-osmotically stimulated was supported by subsequent experiments which showed that iso-osmotic volume expansion with human albumin decreased AVP, and increased water diuresis. Other maneuvers to increase plasma volume in patients with a nephrotic syndrome, such as water immersion and head down tilt, also increased diuresis and natriuresis.
15,
16,
17 The sympathetic nervous system has also been studied in adults with a nephrotic syndrome.
18 Sympathetic nervous system activity was assessed in six patients with a nephrotic syndrome and in six normal control subjects in the supine position. In the patients the plasma norepinephrine levels were elevated, the spillover rate of norepinephrine was markedly increased (0.30 ± 0.07 vs. 0.13 ± 0.02 µg/min/m
2,
P < .05), whereas the norepinephrine clearance rate was comparable to that in the normal subjects (2.60 ± 0.29 vs. 2.26 ± 0.27 L per minute, not significant). Of note, PRA
and plasma aldosterone, AVP, and ANP concentrations were not different in the nephrotic syndrome patients compared with control subjects.
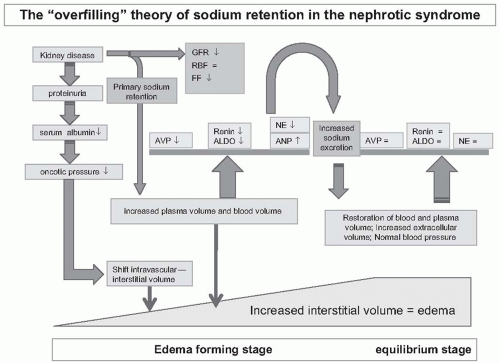
Observations in the seventh and eighth decade of the past century provided arguments against underfilling as the only cause of renal sodium retention in the nephrotic syndrome. Dorhout-Mees et al. initially studied a group of 10 adult patients with minimal change nephrotic syndrome on 13 occasions.
19 The patients were selected for the study because of increased blood volume and blood pressure. Each patient was studied prior to and following prednisone-induced remission. After remission, blood pressure fell in 12 cases, plasma volume fell in 10 cases, and PRA increased in eight cases. Clearly, these data are most compatible with primary overfilling in the nephrotic syndrome (
Fig. 69.2). Data from studies that followed supported the concept of primary renal sodium retention in the nephrotic syndrome. Geers et al. evaluated plasma and blood volume in 88 patients with nephrotic syndrome.
20 Plasma volume was 62.8 ± 9.6 mL per kg lean body mass (LBM) in nephrotic patients and 56 ± 7.1 mL per kg LBM in controls, and blood volume was 94.9 ± 15.1 mL per kg LBM in nephrotic patients versus 88.5 mL per kg LBM in controls. Blood pressures in these and other patients with a nephrotic syndrome were normal or slightly increased.
21,
22 Further evidence to support overfilling comes from studies showing low PRA and PAC in many patients with a nephrotic syndrome.
21,
23,
24 Moreover, neither lowering aldosterone with captopril, blocking aldosterone with spironolactone, nor antagonizing angiotensin II with the analogue saralasin induced natriuresis.
23,
25,
26 It was also questioned if the increased levels of PRA that were observed in some patients with a nephrotic syndrome contributed to sodium retention. Brown et al. evaluated eight patients with a nephrotic syndrome and elevated PRA and PAC.
27 These patients were studied during treatment with captopril, which lowered PAC, and during treatment with intravenous (IV) albumin which decreased both PRA and PAC. Both interventions failed to restore sodium balance. The blood pressure, however, fell with captopril and could have obscured a natriuresis secondary to a decreased PAC.
Additional renal hemodynamic studies and studies of tubular function also supported the overfilling theory. Geers et al. measured GFR using Cr
51-EDTA clearance and ERPF using J
131-hippurate clearance in 41 patients with a nephrotic syndrome.
21 Mean filtration fraction was low, and averaged 14%, arguing against underfilling and a stimulated renin-angiotensin-aldosterone system (RAAS). Detailed clearance studies showed that proximal tubular sodium reabsorption was decreased rather than increased. Usberti et al. studied 21 patients with glomerulonephritis.
28 Tubular glucose reabsorption was used as a marker of proximal tubular sodium reabsorption. The threshold for glucose reabsorption was reduced in the 10 nephrotic patients with edema, suggesting diminished proximal tubular reabsorption. In studies undertaken in five nephrotic patients, a similar conclusion was reached by Grausz et al.
29 In these clearance studies, blockade of sodium reabsorption in the distal nephron with ethacrynic acid and chlorothiazide was used to assess proximal sodium reabsorption. Proximal sodium reabsorption was lower in the nephrotic patients than in normal and in cirrhotic patients.
Studies by Brown et al. and Koomans et al. also provided strong arguments against a role for hypoalbuminemia in the sodium retention of the nephrotic syndrome.
30,
31 These investigators performed detailed clinical observations in patients who were treated with prednisone and developed a remission. In both studies there was a decrease of proteinuria after the start of prednisone. Immediately thereafter sodium excretion increased, well before any noticeable increase of serum albumin levels.
We must be cautious when interpreting the results of the various studies. It is important to consider the timing of the study, the characteristics of the study population, and study methodology. Studies may be done in the edema forming phase, or in the maintenance phase when patients are in equilibrium and many parameters may have normalized (
Fig. 69.1). Patient characteristics include the underlying glomerular disease, the severity of renal injury, the level of GFR, and the rapidity of onset of the nephrotic syndrome. Methodology concerns include the methods used to assess plasma volume and blood volume, and the position of the patient. Measurements of plasma volume and blood volume are imprecise (coefficient of variance 10%). Studies have used different correction factors for plasma and blood volume, using body weight, dry weight, and estimated lean body mass. Plasma volume usually is calculated from the distribution of radioactive labeled albumin. Because the transcapillary escape rate of albumin is increased in patients with a nephrotic syndrome, blood samples must be taken shortly after administration of albumin. Blood volume is calculated from plasma volume or measured red cell mass and hematocrit. However, it is important to note that the ratio of peripheral hematocrit/whole body hematocrit (the so called F cell ratio) is lower in patients with a nephrotic syndrome. If this is not accounted for, calculated blood volume will be overestimated.
Another important issue is the role of body position. Most investigators have performed studies with patients in supine position. However, in patients with a nephrotic syndrome larger changes of plasma volume and blood volume occur upon change of body position. In 1960, Fawcett already studied patients with hypoalbuminemia and edema.
32 In these patients plasma and blood volume decreased to a larger extent compared to control patients as calculated from the change in hematocrit: after 60 minutes of standing hematocrit increased by 12.3 ± 3.4% in patients, and +6.6 ± 2.9% in controls. Similar findings were reported by Eisenberg and Geers.
33,
34 Studies have shown that these changes are relevant, and affect natriuresis. Minutolo studied seven patients with a nephrotic syndrome and evaluated their baseline sodium excretion and the response to IV furosemide while supine and in upright position.
35 In the upright position patients had markedly higher levels of PRA and PAC, and lower sodium and water excretion. Similarly, the response to furosemide was attenuated in the upright position; 6-hour sodium excretion was 40.2 ±
7.8 mmol in the upright position and 64.1 ± 9.1 mmol while supine. Usberti also noted that fractional excretion of sodium was higher when patients were recumbent.
36Finally, interpretation of changes in levels of mediators of neurohumoral activation and effects of any intervention must be done with caution. Activation of PRA and sympathetic nervous system may occur as a consequence of the primary renal disease, and does not necessarily reflect underfilling. In contrast, effects of blockade of aldosterone may be masked by opposite effects of changes in blood pressure.
If we critically review the available literature, it is evident that patients with a nephrotic syndrome may present with characteristics of underfilling or overfilling. In the previously mentioned study of Van de Walle et al. only nine patients had clear signs and symptoms of hypovolemia. Ten other patients had no evidence of hypovolemia. When comparing children with and without hypovolemia, they observed higher PRA and PAC and lower blood volume in hypovolemic patients. These variations in volume status were also seen in children with a nephrotic syndrome caused by renal pathologies other than minimal change disease.
7 Similar observations have been done in adults (
Table 69.2). Usberti et al. described two groups of nephrotic syndrome patients distinguished on the basis of their plasma albumin concentrations.
36 Patients in group 1 had a plasma albumin concentration of less than 1.7 g per dL associated with low blood volumes and atrial natriuretic plasma (ANP) levels, elevated plasma angiotensin II (AT-II) concentrations, and increased proximal tubular reabsorption of sodium (determined by lithium clearance). In contrast, group 2 patients with a plasma albumin concentration greater than 1.7 g per dL exhibited normal blood volumes and plasma hormone concentrations. In all patients blood volume was positively correlated with the plasma albumin concentration, and PRA was inversely correlated with both blood volume and plasma albumin concentration. Of note, GFR was not different between group 1 and group 2 patients (100 ± 25 vs. 101 ± 22 mL per minute, not significant), whereas urinary sodium excretion was substantially lower in group 1 patients (4.88 ± 5.53 vs. 29.9 ± 9.3 mEq per 4 hours,
P < .001). Moreover, acute expansion of blood volume in group 1 patients normalized PRA, plasma AT-II and aldosterone concentrations, fractional sodium excretion, and lithium clearance, while increasing circulating ANP concentrations. Other studies have confirmed these findings, and have added relevant information. Meltzer et al. found that their hypervolemic patients tended to have more severe glomerular involvement, lower GFR, and hypertension.
37 In the study of Geers et al. this variability is also seen.
21 Patients were studied while in sodium balance, and studies were done with patients being recumbent. Overall, plasma volume, blood volume, and blood pressure were normal or above the normal range. There was a striking absence of a correlation between PRA
and blood volume. However, when critically analyzing the data, it is apparent that patients with minimal change disease had lower PV, and higher PRA and PAC. Within the group of patients with minimal change disease, renal impairment was associated with higher blood pressure, PV and blood volume, and lower PRA and PAC.
Thus, patients with nephrotic syndrome can show evidence of underfilling or overfilling. The effective plasma and blood volume in a particular patient will depend on the balance between the (rapidity) of the onset of the nephrotic syndrome, the severity of hypoalbuminemia, and the magnitude of primary renal sodium retention. Thus, underfilling may be more likely in patients with minimal change disease, preserved GFR, and severe hypoalbuminemia (
Table 69.3).
37,
38With respect to the mechanisms of primary renal sodium retention, these have remained largely undisclosed in human studies. The clearance studies have pointed to an intrarenal defect at the level of the distal tubules. Koomans et al. infused albumin in patients with nephrotic syndrome.
39 Patients had increased proximal and distal sodium reabsorption. Infusion of albumin decreased proximal but not distal sodium reabsorption, compatible with a hypovolemia dependent effect on proximal and a primary renal defect of distal sodium reabsorption. In humans, resistance to ANP has been suggested as the culprit. Jespersen studied seven patients with a nephrotic syndrome and 13 age- and sex-matched controls.
40 At baseline, patients had higher blood pressures, lower levels of plasma aldosterone, and higher levels of plasma ANP levels. Both patients and controls received a bolus of 2 ug per kg ANP. Although plasma levels of ANP reached similar levels, sodium excretion was significantly lower in patients. Most importantly, these authors observed that urinary excretion of the second messenger cGMP remained lower in the patients, suggesting a defective ANP signaling. Similar studies were done by Plum et al.
22 These authors studied 31 patients and 10 controls. ANP was infused over 2 hours in 15 patients and 10 controls. At baseline ANP levels were higher in the nephrotic patients. Infusion of ANP increased absolute sodium excretion to a similar extent, in patients and controls. However, sodium excretion factored for the level of ANP was reduced in patients. Again, urinary excretion of cGMP was lower in the patients. Fractional excretion of cGMP was calculated and used as marker of tubular production of cGMP. In the controls fractional excretion of cGMP increased from 93 ± 33% to 159 ± 142%, and in the patients fractional excretion decreased (from 166 ± 77% to 130 ± 58%.), indicating that indeed the tubular production of cGMP was attenuated in the nephrotic syndrome.