Rashad S. Barsoum
The Kidney in Schistosomiasis
Schistosomiasis is a parasitic disease usually acquired by teenagers, often leading to complications that may extend into the fourth and fifth decades of life. It was known to the ancient Egyptians as “the bloody urine disease”1 and is also known as bilharziasis in honor of its discoverer, Theodor Bilharz, the German physician who practiced in Egypt in the 1850s.
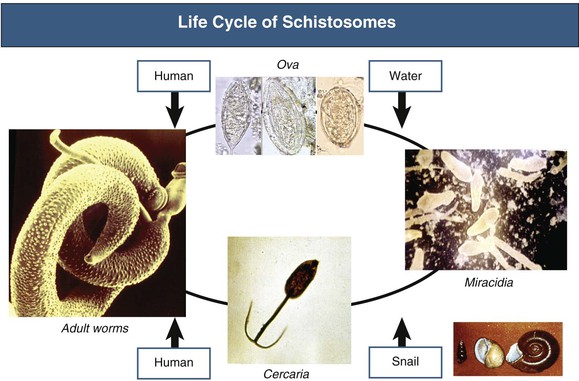
The life cycle of the parasite is shown in Figure 56-1. Infection is acquired through contact with contaminated waters in the ponds and slow-flowing canals originating from certain rivers. Cercariae enter through the skin or mucous membranes and migrate through the lymphatics and blood circulation into the portal or perivesical venous system, where they mature into sexually differentiated adult worms and live in almost continuous copulation. Females leave the males only to lay eggs, traveling against the blood flow, aiming at the rectal or bladder mucosa. The ova are driven out by visceral contraction during defecation or urination in the respective excreta. Contact with fresh water within a couple of days allows the eggs to hatch, releasing miracidia, which infect specific snails. In this “intermediate” host, the organisms mature asexually into cercariae, which are eventually released, searching for their “definitive” host, usually humans and occasionally apes and cattle. The snail demography defines the endemicity and frequency of schistosomiasis in different geographic regions. Because this is largely influenced by climatic factors such as temperature and humidity, it is possible to accurately monitor the global epidemiology of schistosomiasis by satellite remote sensing.2
According to World Health Organization estimates, about 200 million inhabitants of 76 countries on five continents are infected, and an additional 600 million are at risk. Of the infected subjects, 60% are symptomatic, 10% have serious sequelae, and 1% die of the disease each year, mainly in China, the Philippines, Egypt, Brazil, northern Senegal, and Uganda.
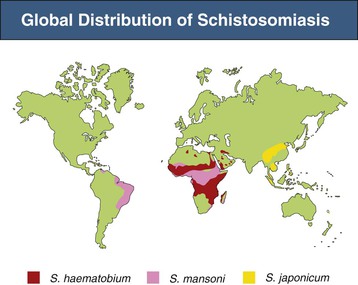
Three species are mainly responsible for almost all the morbidity from the disease, namely, Schistosoma haematobium throughout Africa and adjacent regions; Schistosoma mansoni in Africa, South America, and the Caribbean islands; and Schistosoma japonicum in the Far East (Fig. 56-2). The other four significant human-pathogenic schistosomal species (Schistosoma intercalatum, Schistosoma mekongi, Schistosoma guineensis, and Schistosoma mattheei) have a patchy distribution in certain geographic locations, hence their limited impact on the global epidemiology of schistosomiasis.
S. haematobium affects the urinary tract, whereas S. mansoni and S. japonicum affect the colon and rectum, ultimately reaching the liver and inducing periportal fibrosis. Sporadically, all three species cause “metastatic lesions” when ova are driven by the bloodstream to the lungs, brain, spinal cord, heart muscle, eyes, and other sites.3 Overall morbidity is variable and depends on the virulence of the infective strains, host resistance, environmental factors, and standards of primary medical care. For example, chronic lower urinary tract disease among infected subjects is reported to vary from 2% in Nigeria in the west of Africa to 52% in Tanzania in the east.4
Pathogenesis
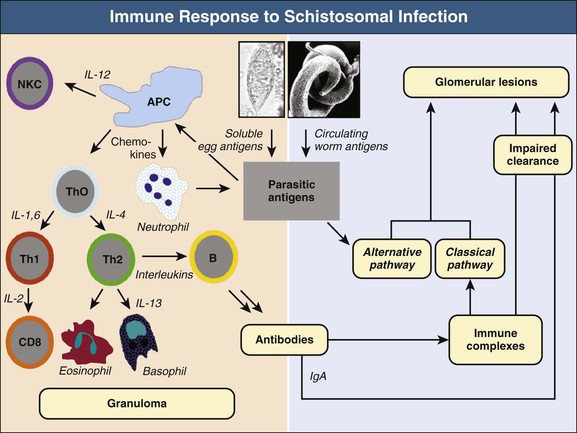
Schistosomes cause morbidity through two major mechanisms: (1) local reactions around the ova deposited in different tissues and (2) systemic effects attributed to the host’s response to circulating antigens released from the worms or the ova (Fig. 56-3).4,5
The local reaction is a cell-mediated immune response to soluble egg antigens diffusing out of trapped ova through micropores in the eggshell. The initial response is innate, being driven by tissue macrophages, and involves natural killer cells, neutrophils, and complement. This response is followed by a specific immune response orchestrated by T helper (Th) lymphocytes.
The schistosomal granuloma is composed of mononuclear cells, eosinophils, neutrophils, basophils, and fibroblasts, which are recruited and activated by a variety of Th lymphokines as well as specific chemoattractants of parasitic origin (Fig. 56-4). These cells are involved in the elimination of the parasite by direct phagocytosis (monocytes), lymphocytotoxicity (T lymphocytes), antibody-dependent cytotoxicity (eosinophils), and antibody- and complement-dependent cytotoxicity (neutrophils). Later, the granuloma is modulated by gradual switching from Th1 to Th2 activation, largely mediated by a change in the monokine profile that favors release of interleukin (IL)–4, which is associated with a phenotypic change of the committed tissue macrophages. At this stage, the intensity of the inflammatory reaction is reduced, and progressive fibrosis is induced largely through the release of IL-4, IL-5, IL-10, somatostatin, and transforming growth factor β. Further on, tolerance to the parasite is achieved by an established population of regulatory T cells, which develop under the combined influence of host- and parasite-derived mediators. With the final extinction of the inflammatory reaction, granulomas in the bladder, lower ureters, and seminal vessels heal with dystrophic calcification.6,7
The systemic immune response is primarily a humoral reaction to circulating schistosomal antigens, which originate mainly from the worm’s digestive enzymes (gut antigens), with a minor contribution from the tegument and ova. The gut antigens consist of a positively charged glycoprotein and a negatively charged proteoglycan (circulating cationic antigens and circulating anionic antigens, respectively). These antigens are present in most of the schistosomal immune complex–mediated lesions, particularly in glomeruli.8 The antibody response is biphasic, reflecting the successive Th1 and Th2 stages of lymphocyte activation. In the Th1 stage, B cells tend to synthesize IgM, IgG1, and IgG3 under the influence of IL-2. During the Th2 phase, IgG2, IgG4, and IgA predominate; these have a limited ability to fix complement and may even block its deposition, hence their importance in modulating the granulomas.4,6
Clinical Manifestations
Schistosomal lesions in the urinary system mirror the two major pathogenetic mechanisms. On one hand, there are local lesions, mostly affecting the lower urinary tract, caused by the local granulomatous response to S. haematobium ova.9 On the other hand are those lesions caused by immune complex deposition in the glomeruli, usually associated with hepato-intestinal S. mansoni and, less commonly, urogenital S. haematobium infections.10 Although glomerular lesions can be readily induced by S. japonicum in experimental animals,11 this species does not seem to cause significant kidney disease in humans.
Lower Urinary Tract Schistosomiasis
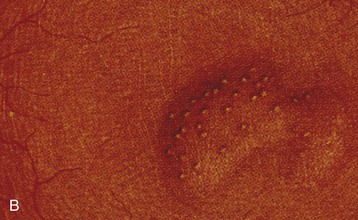
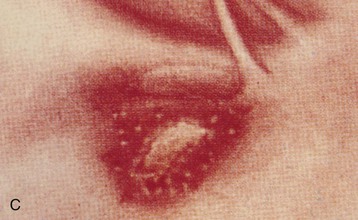
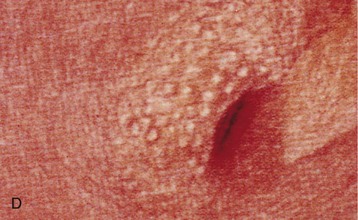
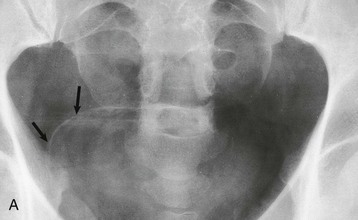
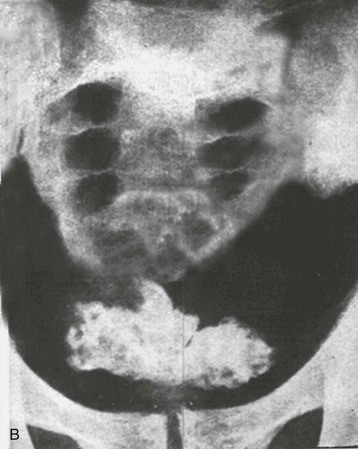
The lower urinary tract and adjacent genital structures are the primary sites of S. haematobium infection. Clinical disease starts by the coalescence of multiple granulomas that form small “pseudotubercles” in the bladder mucosa (Fig. 56-5). These may consolidate to form sessile, occasionally pedunculated masses or ulcerate, leading to painful terminal hematuria, the most typical presenting symptom. Hematuria may vary from microscopic (40% to 100% in different reports) to gross (0% to 97%).12 Ulcers eventually heal by fibrosis, with calcified granulomas under the atrophic and dirty mucosa, leading to the characteristic cystoscopic appearance of sandy patches and also the radiologic appearance of linear bladder calcifications in 2% to 62% of cases.12 Similar lesions may occur in the lower ureters, bladder neck, seminal vesicles, and other organs in the vicinity (Fig. 56-6).
The bladder lesions predispose to secondary bacterial infection, particularly with Pseudomonas or Proteus species, usually following instrumentation. Proteus infection is notorious for favoring stone formation, which further complicates the scenario. The subsequent fibrotic process may involve the bladder neck, leading to outflow obstruction, or the vesicoureteral junction, leading to ureteral obstruction or vesicoureteral reflux. Involvement of the detrusor is a late event that may interfere with the motor function of the bladder, leading to an atonic or a hyperirritable viscus. Eventually, the bladder becomes a deformed, contracted, and calcified organ that accommodates a very small amount of urine that it can hardly void.
Bladder Cancer
Chronic bilharzial cystitis is precancerous. In a study of almost 10,000 Egyptian patients with bladder cancer, schistosomal association was confirmed in 55.3%. The predominant lesions were transitional cell carcinoma (65.8%) and squamous cell carcinoma (28.4%).13 The tumor, particularly when of the squamous cell type, remains localized for a long time before spreading to the surrounding pelvic tissues or a distant site, thanks to the occlusion of lymphatics by the preceding fibrotic process.
Associated infection with human papillomavirus is encountered in about one fourth of cases, which suggests an etiologic role in malignant transformation.14 Specific p53 gene mutations were detected in one third of cases.15 This may be attributed to neutrophil-generated reactive oxygen molecules, cleavage of conjugated urinary carcinogens, or production of nitrosamines by bacterial enzymes.16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree