Chapter 66 TENSION-FREE CYSTOCELE REPAIR USING PROLENE MESH
Pelvic organ prolapse (POP) is one of the emerging problems in women, considering the aging population in Western countries together with already known risk factors such as race, genetic predisposition, pregnancy, parity, obstetric trauma, menopause, and previous gynecologic surgery. Among these factors, certainly white Caucasian women have a higher odds ratio (1.0) with respect to African American and Hispanic women (0.6 and 1.2, respectively).1
Considering the different segments involved in the development of POP, the anterior vaginal wall, and in particular the bladder, represented the site most frequently affected in a study of 27,000 American women; the posterior segment (rectocele) was present in 19%, and uterine prolapse in 14%.1
Mant and colleagues, in 1997, studied a group of 17,000 women and observed that the incidence of anterior colporrhaphy was 37%, compared with a 24% rate of vaginal hysterectomy.2
Numerous surgical procedures have been proposed in the last century to correct all types of anterior vaginal wall defects (central, paravaginal, combined), using either an abdominal or vaginal approach. Despite improved understanding of pelvic anatomy and organ function and the advancement of surgical techniques, the long-term success rate is still variable. Recurrence rates for the various surgical procedures used to treat anterior vaginal prolapse range from 3%3 to 20%4 after single anterior colporrhaphy, from 22%5 to 92%6 if the anterior colporrhaphy is combined with other procedures (sacrospinous ligament suspension), from 2%7 to 59%8 after four-corner suspension, and from 5%9 to 50%10 after vaginal paravaginal repair. The reasons for such discrepancies are several and include poor patient selection, suboptimal surgical technique, inappropriate choice of suture material, and persistence of predisposing risk factors for prolapse (constipation, chronic respiratory disease).11 Moreover, Farrell and colleagues emphasized that the very existence of the pubocervical fascia used during anterior colporrhaphy is doubtful. They observed in samples obtained during surgical procedures for the correction of anterior defect that the so-called fascia is adequately identified only 58% of the time and concluded that this finding calls into question techniques that rely on isolated fascia for the repair of anterior and posterior defects.12
EVOLUTION OF SURGICAL PROCEDURES
In the last 20 years, the repair of anterior vaginal wall prolapse has shifted from a classic anterior repair to a more detailed surgical procedure (site-specific repair) with an attempt to identify the precise localization of the tissue defect. For example, Richardson and associates proposed the vaginal paravaginal repair using native tissues to restore the anatomy.13 These concepts were further explored in surgery of the posterior compartment. However, despite encouraging results, the anterior segment (i.e., the connective tissue supporting the bladder) remained the site of least durability.14,15
CONNECTIVE TISSUE AND ITS ROLE IN THE PELVIC FLOOR
Elastin and laminin are two other glycoproteins that may play a role in elasticity of the ground substance. Elastin provides the ability of a tissue to be stretched and is found not only in the bladder as a component of compliance but also in the urethra. Elastin is degraded by elastase, which is part of a proteaseantiprotease control mechanism found in the plasma. It has recently been demonstrated that there is an increase in the percentage activity of elastase in women with stress urinary incontinence (SUI) compared with aged-matched controls.16 Several factors, such as age, stress, hormones, and growth factors, are involved in the regulation of connective tissue metabolism.17
Collagen turnover slows down with increasing age, and the concentration increases after menopause.18–21 The changes during menopause seem to generate a connective tissue with a higher collagen concentration but with alterations in cross-linking,22 and there is also an increase in fibril diameter in the cardinal and uterosacral ligament in patients with POP and SUI. This produces a tissue with an increased load-bearing ability but decreased elasticity.23 Based on biochemical assays, Sayer and colleagues24 reported modification of cross-linking in women with bladder neck prolapse and SUI. A reduction of collagen in the pubocervical fascia25 and in nonsupportive pelvic tissue has also been observed.26 Recent studies indicate that women of fertile age with SUI demonstrate changes in the extracellular matrix of the paraurethral connective tissue resulting in a stiffer and less supportive connective tissue and a defect in the connective tissue glue.27–30 In women with genital prolapse after menopause, there seems to be a difference in collagen metabolism suggesting a change in the degradation of collagen related to matrix metalloproteinases and with a significant reduction in the ratio of type I to type III collagen.31 Therefore, it is not the amount of collagen but the amount of fresh elastic collagen that is important for the mechanical strength of supporting fascia layers.
HEALING PROCESS AFTER SURGICAL REPAIR
When a wound is created, such as an anterior vaginal wall and pubocervical fascia incision, fibrous protein synthesis and remodeling reestablish tissue strength. Collagen is a central factor in wound healing. The first day after injury, immature fibroblasts synthesize and secrete collagen and proteoglycans. A weaker and more elastic flexible type III collagen is the principal type found in early wounds. After 2 weeks, the majority of regenerated tissue is composed of type III collagen, which accounts for only 7% of its final tensile strength. With maturation of the scar, a stronger type I gradually replaces the type III collagen. In this way, an organized cross-linked pattern regains some tensile strength, but the new tissue is never as strong as the original tissue.32 The factors that influence healing and scar maturation are still incompletely understood, even though we know that the gene for type III collagen seems to be activated more than the gene for type I collagen.33
WHAT HAPPENS WHEN A MESH IS IMPLANTED IN THE BODY
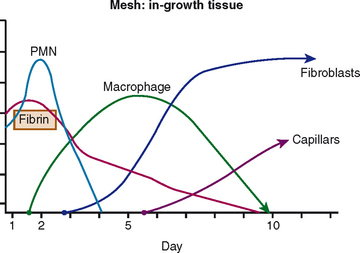
These events happen as a result of the interaction between the graft materials (biologic or synthetic) and the tissue interface, which is of prime importance; for this reason, we need to reduce the intensity and time of the inflammatory period, in particular with regard to reducing potential complications (erosion and extrusion) (Fig. 66-1).
Once the prosthetic material has been implanted, there are four types of tissue reactions: minimal response with a thin layer of fibrosis around the implant; chemical response with a severe and chronic inflammatory reaction around the implant; physical response with an inflammatory reaction to certain materials and the presence of giant cells; and necrotic tissue, a layer of necrotic debris produced as a result of in situ exothermic polymerization.34
PROPERTIES OF SYNTHETIC BIOMATERIALS
Biocompatibility is the capability of a material to provoke a favorable reaction when introduced into a living system. Consequently, a biomaterial is any material, natural or man-made, or any biomedical device that performs, augments, or replaces a natural function. One definition of biocompatibility is “the utopian state where a biomaterial presents an interface with a physiologic environment without the material adversely affecting that environment or the environment adversely affecting the material.” The biomaterials employed can come close to this utopian state, but we must accept compromises between the benefits and the undesired reactions caused to the living system by the material.
Cumberland35 and Scales36 delineated a series of properties of an “ideal” synthetic biocompatible materials:
At the present time none of the synthetic meshes meet all of these criteria.
Based on their pore size, the most common synthetic meshes can be classified into four types:
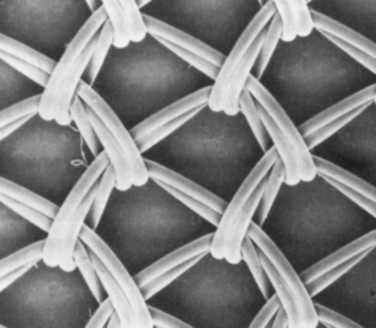
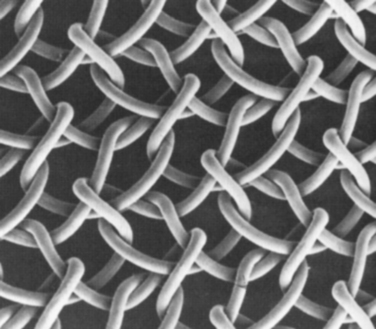
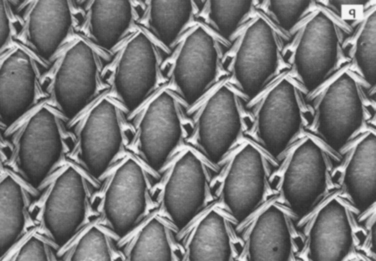
Another important property is the composition of fibers (Table 66-1). Polypropylene meshes are monofilaments, whereas the other commonly used meshes are multifilaments. One theoretical disadvantage of multifilament meshes is that the interstices are smaller than 10 μm; this allows small bacteria to infiltrate and proliferate, but macrophages and neutrophilic granulocytes that could eliminate the bacteria are too large to enter a 10-μm tridimensional pore.39–41 In addition, because of their wide pores, type I materials (but not types II and III) allow rapid in-growth of vascularized fibrous tissue, as was demonstrated by Chvapil and colleagues42; this leads to fibroplasia and angiogenesis, which not only prevent infection but also form fibrous connections to the surrounding tissue. Therefore, pore size is a key factor in determining inflammatory response and the growth of fibrocollagenous tissue.
Table 66-1 Composition and Types of Fibers
| Chemical Component | Trade Name (Manufacturer) | Type |
|---|---|---|
| Polypropylene | Marlex (CR Bard, Branston, RI) | Monofilament |
| Prolene (Ethicon, Sommerville, NJ) | Monofilament | |
| Atrium (Atrium Medical, Hudson, NH) | Monofilament | |
| Polytetrafluoroethylene (PTFE) | Teflon (CR Bard, Haverhill, MA) | Multifilament |
| Expanded PTFE | Gore-Tex (WL Gore, Flagstaff, AZ) | Multifilament |
| Polyethylene tetraphthalate | Mersilene (Ethicon, Somerville, NJ) | Multifilament |
| Polyglycolic acid | Dexon (absorbable) (Davis and Geck, American Cyanamid, Danbury, CT) |