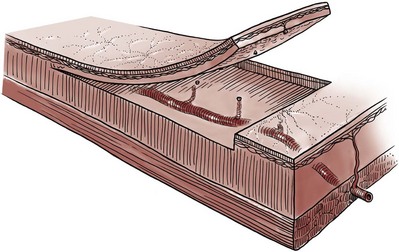
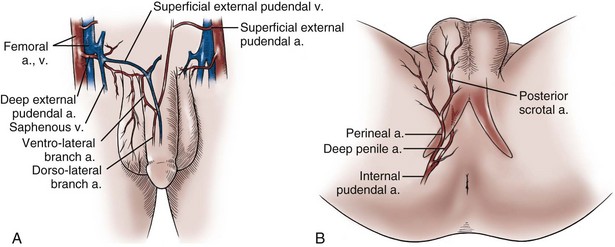
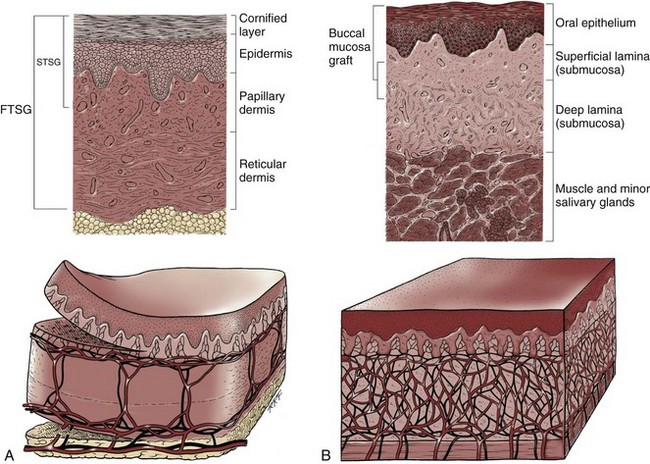
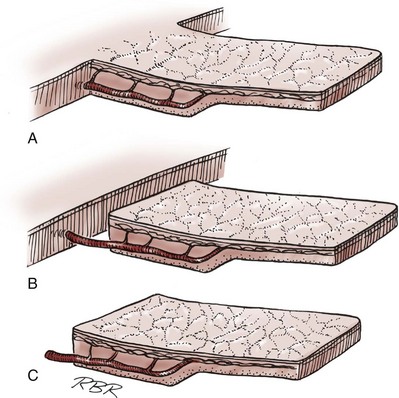
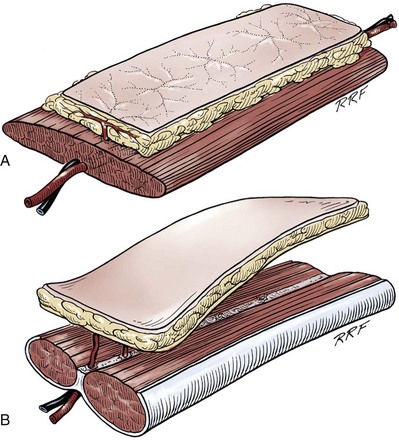
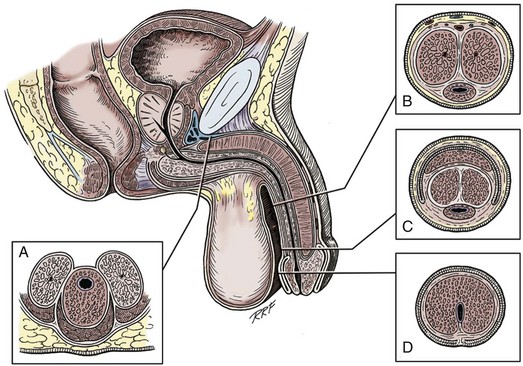
Gerald H. Jordan, MD, FACS, FAAP (Hon), FRCS (Hon), Kurt A. McCammon, MD, FACS Tissue can be transferred as a graft (Fig. 36–1A and B). The term graft implies that tissue has been excised and transferred to a graft host bed, where a new blood supply develops by a process termed take. Take requires approximately 96 hours and occurs in two phases. The initial phase, imbibition, requires about 48 hours. During that phase, the graft survives by “drinking” nutrients from the adjacent graft host bed, and the temperature of the graft is less than the core body temperature. The second phase, inosculation, also requires about 48 hours and is the phase in which true microcirculation is reestablished in the graft. During that phase, the temperature of the graft rises to core body temperature. The process of take is influenced by both the nature of the grafted tissue and the conditions of the graft host bed. Processes that interfere with the vascularity of the graft host bed thus interfere with graft take. (From Jordan GH, Schlossberg SM. Using tissue transfer for urethral reconstruction. Contemp Urol 1993;13:23.) Thus if a graft is a split-thickness unit, that graft carries the epidermis or the covering. That graft also exposes the superficial dermal (intradermal or intralaminar) plexus. In most grafts, that superficial plexus is composed of small but numerous vessels. This thus conveys favorable vascular characteristics to a split-thickness unit. The unit has few lymphatics, and the physical characteristics are not carried, which accounts for the tendency of split-thickness units to be brittle and less durable. The reticular dermis is not carried with the split-thickness unit (Jordan, 1993). The mesh graft is usually an application of the split-thickness graft. After the harvest of a sheet graft, the sheet is placed on a carrier that cuts systematically placed slits in the graft. These slits can expand the graft by various ratios (i.e., 1.5 : 1, 2 : 1, 3 : 1). For most genital reconstructive surgery, the slits are not for expansion but rather to allow subgraft collections to escape; in some cases, the slits allow the graft to conform better to irregular graft host beds (e.g., the testes in split-thickness skin graft scrotal construction). It has also been proposed that mesh grafts take readily because of increased levels of growth factors, possibly as a function of the slits. In general, full-thickness skin grafts are not meshed (Schreiter and Koncz, 1983; Jordan, 1993). If a graft is a full-thickness unit, it carries the covering and the superficial dermis or lamina with all the characteristics attributable to that layer. It also, however, carries the deep dermis or deep lamina. In skin, the subdermal plexus is exposed. In most cases, that plexus is composed of larger vessels that are more sparsely distributed. The graft is thus fastidious in its vascular characteristics. A full-thickness unit carries most of the lymphatics, and the physical characteristics are likewise carried with the transferred tissue (Devine et al, 1976; Jordan, 1993; Wessels and McAninch, 1996). An off-the-shelf substance called Integra is currently available. Integra (Integra LifesSciences Corporation, Plainsboro, NJ 08536, 1-877-444-1122) is used to “prepare” graft recipient sites. After a period, the Integra material becomes infiltrated with granulation tissue and after about 10 days to 2 weeks can be grafted with split-thickness skin. The resultant graft is said to have both a dermal layer as well as the graft covering. We have limited experience with the material at this time. When the grafts that are most commonly used in genitourinary reconstructive surgery are examined, the split-thickness skin graft has favorable vascular characteristics but tends to contract and be brittle when mature. The full-thickness skin graft tends to have more fastidious vascular characteristics, but it does not contract as much and is more durable when mature (see Fig. 36–1A). There is a difference between genital full-thickness skin (penile and preputial skin grafts) and extragenital full-thickness skin. This is probably a reflection of the increased mass of the graft in extragenital skin grafts. This increased mass makes the graft more fastidious, and the poor results reported with urethral reconstruction with extragenital full-thickness skin grafts are probably due to poor or ischemic take (Webster et al, 1984; Webster, 1987; Jordan, 1993). The posterior auricular graft (Wolfe graft) is an exception to the rule concerning extragenital skin. The postauricular skin is thin and overlies the temporalis fascia and is thought to be carried on numerous perforators. The subdermal plexus of this graft thus mimics the characteristics of the intradermal plexus, and the total mass of the graft is more like that of the split-thickness unit. In the bladder epithelial graft, there is a superficial and a deep plexus; however, the plexuses are connected by many more perforators. Thus bladder epithelial grafts tend to have more favorable vascular characteristics. In the case of the oral mucosal grafts, there is a panlaminar plexus. Thus the oral mucosal graft can be thinned somewhat, provided a sufficient amount of deep lamina is carried to preserve the physical characteristics (see Fig. 36–1B). The oral mucosal grafts are thought to have optimal vascular characteristics (Humby, 1941; Memmelaar, 1947). The thinned graft diminishes the total graft mass while preserving the physical characteristics and not adversely affecting the vascular characteristics. The enthusiasm for the buccal mucosal graft thus seems well founded. The fact that the graft has a “wet epithelial” surface is likewise thought to be a favorable characteristic for many cases of urethral reconstructive surgery. The lingual, labial, and buccal grafts all vary in thickness and somewhat in substance. Because the labial mucosal grafts are thin, many surgeons prefer that donor site for reconstruction of the fossa navicularis (Jordan, 1993). A series reporting the use of “buccal mucosal” onlay grafts with mid- and long-term results seems to suggest durability for these grafts. In that series (Fichtner et al, 2004), 67 patients were described, all with follow-up exceeding 5 years and some with 10 years of follow-up. All failures occurred within 12 months of the original procedure. The dermal graft has been used for years to augment the tunica albuginea of the corpora cavernosa. When it is harvested, the graft exposes both the intradermal plexus and the deep dermal plexus. The dermal graft thus takes readily (is not fastidious) and has the physical characteristics normal to skin. When it is properly prepared, the tunica vaginalis graft is essentially peritoneum. The tendency of peritoneum to take readily is well documented both in the literature that examines adhesion formation and in the urology literature concerning the application of peritoneal grafts for reconstruction of the urinary tract. The literature fails to accurately define what the surgeon can expect regarding physical characteristics (Jordan, 1993). Tunica vaginalis grafts have proved useful for small defects of the tunica albuginea of the corpora cavernosa, but there is a tendency to aneurysmal dilation when they are used for larger defects. Tunica vaginalis grafts have been tried for urethral reconstruction with uniformly poor results. As described in the urologic literature, vein grafts are perhaps not true grafts according to the terminology used in this chapter. Vein patches are widely used in vascular surgery. The premise is that the vein survives by endothelial direct perfusion and reestablishment of vein wall blood flow by perfusion of the vasa vasorum. The vascular literature is at odds with this concept. The intima is the endothelial layer; it is thin and easily injured during the process of vein harvest and preparation, with areas of endothelial sloughing noted. Inflammatory cells and fibrin adhere to the exposed basement membrane. However, the endothelium does regenerate in the first 6 weeks. The media is a combination of smooth muscle and interlaced collagen. After graft harvest, smooth muscle injury is prominently noted and is thought to be related to warm ischemia. In more mature grafts, much of the smooth muscle is replaced by a process of fibrous transformation with collagen deposition. The adventitia is a loose collagenous network interspersed with vasa vasorum. Mature vein grafts show evidence of take to the vasa vasorum. However, the adventitia becomes incorporated by periadventitial connective tissues. Thrombosis in the vasa vasorum, early in the process of take, is not an unusual phenomenon. When vein grafts are exposed to arterial pressure and shear stress forces, the process colloquially described as “arterialization” occurs and is associated with changes of the vessel wall elastic properties, and the graft becomes rigid with low compliance. Once these changes are noted, at least when veins are used for vessel replacement, the graft remains noncompliant throughout the remaining life of the graft (Szilagyi et al, 1973; Fuchs et al, 1978; Tolhurst and Haeseker, 1982). Vein “grafts” are currently being widely used for replacement of defects of the tunica albuginea of the corpora cavernosa. The pertinent points with regard to the transfer of vein patches to the corpora cavernosa and their long-term behavior, however, have been inferred from the current vascular literature. Dermal grafts have been tried for urethral reconstruction, also with poor results in general. Rectal mucosal grafts have also been proposed for urethral reconstruction but little is known about their graft take. In general, the vascularity of the bowel mucosa is based on the vascularity of the underlying muscle, with the mucosa carried on perforators. Little is found in the literature regarding the process of take of these grafts. Tissue can be transferred as a flap. The term flap implies that the tissue is excised and transferred with the blood supply either preserved or surgically reestablished at the recipient site. Flaps can be classified by a number of criteria. We can classify flaps on the basis of their vascularity and thus characterize flaps as either random flaps (Fig. 36–2) or axial flaps (Fig. 36–3). A random flap is a flap without a defined cuticular vascular territory. The flap is carried on the dermal or laminar plexuses; the dimensions of random flaps can vary widely from individual to individual and from body site to body site. The term axial flap means that there is a defined vessel in the base of the flap. There are three types of axial flaps. The direct cuticular axial flap is a flap based on a vessel superficial to the superficial layer of the deep body wall fascia (see Fig. 36–3A). The classic example of a direct cuticular flap is the groin flap. A musculocutaneous flap (Fig. 36–4A), on the other hand, is based on the vascularity to the muscle. The overlying skin paddle is carried on perforators. If the muscle alone is carried as a flap, the overlying skin survives as a random unit. The fasciocutaneous system of vascularity (Fig. 36–4B) is similar to the musculocutaneous system. However, the deep blood supply is carried on the fascia (both deep and superficial layers), and the overlying skin paddle is based again on perforators. Thus one can transfer a fascial flap based on the deep blood supply associated with the flap; the overlying skin, if it is not carried with the flap, remains as a random unit (Ponten, 1981; Tolhurst and Haeseker, 1982; Cormack and Lamberty, 1984). It has been argued that fascia is relatively avascular and hence cannot serve as “the blood supply” to the fasciocutaneous unit. Indeed, the fascial layer, in reality, acts as a trellis—thus the vessels are carried much like the limbs of a vine (Jordan, 1993). (A to C, From Jordan GH, McCraw JB. Tissue transfer techniques for genitourinary reconstructive surgery. AUA Update Series 1988;7:lesson 10.) (A and B, From Jordan GH, McCraw JB. Tissue transfer techniques for genitourinary reconstructive surgery. AUA Update Series 1988;7:lesson 10.) A flap can also be classified by the elevation technique. A peninsular flap is a flap in which the vascular continuity and the cutaneous continuity of the flap base are left intact (see Figs. 36-2 and 36-3A). An island flap (see Fig. 36–3B) is a flap in which the vascular continuity is maintained; however, the cuticular continuity is divided. Thus a true island flap is elevated on dangling vessels. The microvascular free transfer flap (free flap) (see Fig. 36–3C) has both the vascular continuity and the cuticular continuity interrupted. The vascular continuity is then reestablished at the recipient site. Again, there is confusion in terminology. In genitourinary reconstructive surgical procedures, we tend to use the term island flap. As already mentioned, a true island flap is elevated on dangling vessels. The usual case, however, is that a skin island or paddle is elevated either on the muscle, as in the gracilis musculocutaneous flap, or on the fascia, as in local genital skin flaps. The term island flap is not synonymous with the terms skin island and skin paddle. The usefulness of these flaps and grafts is illustrated in the discussion of the surgical techniques in this chapter. There is continued interest in the use of tissue-cultured grafts or “manufactured” grafts. The likelihood of someday, soon, being able to successfully use off-the-shelf grafts or sheets of cultured material is right around the corner (Chen et al, 1999; Atala, 2002; Rotariu et al, 2002; El-Kassaby et al, 2003; Bhargava et al, 2004). Key Points: Principles of Reconstructive Surgery See Figs. 36-5 to 36-13 on the Expert Consult website The anatomic relationships of the male genitourinary structures in the penis and male perineum must precede the discussion of specific reconstructive surgical techniques; for a complete anatomic description please see Chapter 2. The penile shaft (Fig. 36–5) comprises three erectile bodies: the two corpora cavernosa and the corpus spongiosum containing the urethra, along with their enveloping fascial layers, nerves, and vessels, all covered by skin. All these structures continue into the perineum. The corpora cavernosa contain erectile tissue within a dense elastic sheath of connective tissue called the tunica albuginea. The corpora cavernosa are not separate structures but constitute a single space with free communication through an incompetent midline septum that becomes more complete toward the base of the penis. This erectile tissue contains arteries, nerves, muscle fibers, and venous sinuses lined with flat endothelial cells, and these features fill the corpora cavernosa, making its cut surface look like that of a sponge. This tissue is separated from the tunica albuginea by a thin layer of areolar connective tissue. The third erectile body, the corpus spongiosum, lies in the ventral groove between the two corpora cavernosa. The tunica albuginea (adventitia) of the corpus spongiosum is thinner than the tunica albuginea of the corpora cavernosa, and the corpus spongiosum contains less erectile tissue than the corpora cavernosa. The urethra traverses the length of the penis within the corpus spongiosum. At its distal end, the corpus spongiosum expands to form the glans penis. The urethral meatus is slitlike, lying slightly on the ventral aspect of the tip of the glans, with its long axis oriented vertically. At its base, the penis is supported by two ligaments, composed primarily of elastic fibers that are continuous with the fascia of the penis. Posterior to this attachment, the right and left corpora cavernosa diverge, and the corpus spongiosum broadens between the two crura to form the bulbospongiosus (bulb) (Fig. 36–6). (A to D, From Jordan GH. Complications of interventional techniques of urethral stricture disease: direct visual internal urethrotomy, stents and laser. In: Carson C, editor. Topics in clinical urology: complications of interventional techniques. New York: Igaku-Shoin; 1996. p. 86–94.) Figure 36–5 also illustrates the relationship of the erectile bodies and the urethra to the structures in the perineum. For the discussion of trauma and reconstruction, it is the consensus opinion of a World Health Organization conference convened in Stockholm in 2002 that the common use of the terms anterior urethra and posterior urethra be put aside and that the urethra be subdivided into six separate areas. These portions of the urethra are illustrated in Figure 36–7. (Modified from Devine CJ Jr, Angermeier KW. Anatomy of the penis and male perineum. AUA Update Series 1994;8:11.) A submucosal layer is noted throughout the length of the urethra. Five “sphincters” are recognized (Fig. 36–8). In the penis, the erectile bodies are surrounded by Buck fascia, dartos fascia, and skin. Buck fascia is the tough, elastic layer immediately adjacent to the tunica albuginea (see Fig. 36–5). On the superior aspect of the corpora cavernosa, the deep dorsal vein, paired dorsal arteries, and multiple branches of the dorsal nerves are contained within the envelope of Buck fascia. In the midline groove on the underside of the corpora cavernosa, Buck fascia splits to surround the corpus spongiosum. Consolidation of the fascial layers (Fig. 36–9A to C), lateral to the corpus spongiosum, attaches it to the tunica albuginea of the corpora cavernosa. Attached distally to the undersurface of the glans penis at the corona, Buck fascia extends into the perineum, enclosing each crus of the corpora cavernosa and the bulb of the corpus spongiosum, and firmly fixing these structures to the pubis, ischium, and inferior fascia of the perineal membrane (urogenital diaphragm). Blood is supplied to the skin of the penis by the left and right superficial external pudendal vessels (Fig. 36–10A), which arise from the first portion of the femoral artery, cross the upper medial portion of the femoral triangle, and divide into two main branches, running dorsolaterally and ventrolaterally in the shaft of the penis, with collateralization across the midline. At intervals, fine branches split off to the skin, forming a rich subdermal vascular plexus that can sustain the skin after its underlying dartos fascia has been mobilized. The arteries are accompanied by venous tributaries that are more prominent and easily seen than the arteries. Because of its remarkable thinness and mobility, and the character of its vascular supply, the skin covering the penis is an ideal substitute—in some cases, for urethral reconstruction. The blood supply to the scrotal wall and ventral penile skin is based on the posterior scrotal artery, a superficial vessel from the deep internal pudendal artery (Fig. 36–10B). As with the superficial external pudendal tributaries, the posterior scrotal system provides a series of tributaries carried within the tunica dartos. The penis is drained by three venous systems: superficial, intermediate, and deep (Fig. 36–11) (Aboseif et al, 1989). The superficial veins contained in the dartos fascia on the dorsolateral aspects of the penis unite at its base to form a single superficial dorsal vein. The superficial dorsal vein usually drains into the left saphenous vein (rarely into the right), and occasionally forms two trunks that drain into both. Veins from more superficial tissue may drain into the external superficial pudendal veins. Figure 36–11 Diagram illustrates the venous drainage of the deep structures of the penis. (From Horton CE, Stecker JF, Jordan GH. Management of erectile dysfunction, genital reconstruction following trauma and transsexualism. In: McCarthy JG, editor. Plastic surgery, vol 6. Philadelphia: WB Saunders; 1990. p. 4213–45.) The blood supply to the deep structures of the penis is derived from the common penile artery, which is a continuation of the internal pudendal artery after it gives off the perineal branch (Fig. 36–12). From that point, the artery is termed the common penile artery and travels along the medial margin of the inferior pubic ramus. As it nears the urethral bulb, the artery divides into its three terminal branches, as follows: Figure 36–12 Diagram illustrates the arterial supply to the deep structures of the penis. (From Horton CE, Stecker JF, Jordan GH. Management of erectile dysfunction, genital reconstruction following trauma and transsexualism. In: McCarthy JG, editor. Plastic surgery, vol 6. Philadelphia: WB Saunders; 1990. p. 4213–45.) The perineum is the diamond-shaped outlet bounded anteriorly by the pubic arch and the arcuate ligaments of the pubis, posteriorly by the tip of the coccyx, and laterally by the inferior rami of the pubis and ischium. A transverse line between the ischial tuberosities divides the perineum into an anterior triangle containing the external urogenital organs and a posterior anal triangle (see Fig. 36–12A and B). In the anterior triangle, Colles fascia (Fig. 36–13A) attaches at its posterior margin to the perineal body at the posteroinferior margin of the urogenital diaphragm. The fascia curves below the superficial transverse perinei muscles and projects forward as two layers attached laterally to the ischium and the inferior ramus of the pelvis. The loose superficial layer is fatty and is continuous with the more substantial dartos fascia (tuncia dartos) of the scrotum. In the scrotum, the dartos fascial layer contains muscle fibers that cause the rugous appearance of the scrotum. The fascia also projects (but without muscle fibers) into the midline, to form the septum between the halves of the scrotum. The median raphe in the skin delineates the separation of the halves of the scrotum and is continued anteriorly as a darkly colored streak in the ventral midline of the penis and posteriorly as the median raphe of the perineum terminating at the anus. (A to D, From Devine Jr CJ, Angermeier KW. Anatomy of the pelvis and male perineum. AUA Update Series 1994;13:1015.) Anteriorly, Colles fascia fuses and becomes continuous with the membranous layer of the subcutaneous connective tissue of the anterior abdominal wall (Scarpa fascia). Laterally, Colles fascia fuses to the pubic arch and with the fascia lata. Posteriorly, Colles fascia sweeps beneath the transverse perinei muscles, fusing with the posterior aspect of the perineal membrane. The space beneath the continuous plane formed by these fascial attachments is the superficial perineal pouch, in which infections or extravasation of urine and collections of blood (after trauma to the urethra) may be confined (see Fig. 36–9A to C). In males, the superficial perineal space contains the continuation of the corpora cavernosa, the proximal part of the corpus spongiosum and urethra, the muscles associated with them, and the branches of the internal pudendal vessels and pudendal nerves (Fig. 36–13B). Lying just anterior to the anus, as a part of the plane separating the anterior and posterior perineal triangles, the perineal body is formed by the interconnection of eight muscles of the perineum (see Fig. 36–13A and B). The perineal body receives fibers from the anterior portion of the anal sphincter and is the central point of insertion of the superficial transverse perinei muscles that arise at the ischial tuberosities. The bulbospongiosus muscle (midline fusion of the ischiocavernosus muscle) is fixed to the perineal body by its most posterior fibers. The deep transverse perinei muscles and fibers from the anterior portions of the levator ani muscles attach to the deep aspect of the perineal body. The urogenital diaphragm constitutes the deep perineal space (see Fig. 36–13C and D). It is contained within two layers of fascia and incompletely covers the outlet of the pelvis anterior to the deep layer of the perineal body. The deep layer of fascia is an indistinct structure—the continuation of the endopelvic obturator fascia. The superficial fascia attaches laterally to the ischial rami and the inferior ramus of the pubis. This fascia blends with the deep layer behind the perineal body and anteriorly, where it terminates with a thickened edge, the transverse perineal ligament. A space between this ligament and the arcuate ligament of the pubis accommodates the deep dorsal vein of the penis. The deep perineal pouch (see Fig. 36–13D) contains the deep transverse perinei muscles, the external sphincter of the urethra, the bulbourethral (Cowper) glands, and the blood vessels and nerves associated with the structures within it. The sphincter urethral muscle fibers arise from the medial surface of the inferior pubic rami and pass medially toward the urethra, where they meet the fibers from the opposite side. In males, the muscle encircles the membranous urethra to function as the somatic sphincter of the urethra (Haertsch, 1981). Surgical position and retraction are critical to attaining good results. If possible, procedures are done with the patient supine or prone. Many procedures that previously were done with the patient in the lithotomy position can be done with the patient in the frog-leg or split-leg position. For penile surgery, a Scott retractor with stay hooks,* the Jordan-Bookwalter perineal retractor set,† or the Omni-Tract perineal retractor‡ are helpful. Lithotomy or exaggerated lithotomy positions are used only for the minimal time necessary. With appropriate padding for the foot and positioning without pressure on the back of the leg, the complications in the low lithotomy position are minimal. When the patient is in the supine, split-leg, and low lithotomy positions, venous compression stockings can be used. The controversy in positioning revolves around the use of the exaggerated lithotomy position. It is our preference to use this position for all bulbar and posterior urethral reconstructions. Other surgeons use a lower lithotomy position. We find the more exaggerated position to be safe and believe that it provides unequaled access to the deep perineal structures (Angermeier and Jordan, 1994). Details of positioning, as we do it, are covered later. To minimize the patient’s time in the exaggerated position, all graft harvesting or flap elevation is done with the patient in the flat supine position. Key Points: Reconstructive Surgical Techniques Lichen sclerosus (LS) is the preferred term for what was previously known as balanitis xerotica obliterans (BXO). Lichen sclerosus is a chronic inflammatory disorder of the skin of uknown origin. No specific mechanism of disease has been elucidated. There are several acquired scarring disorders of the skin associated with pathology of the basement membrane, such as mucous membrane pemphigoid, that may be shown as related (Bernard et al, 1992; Akporiaye et al, 1997). The reported incidence of LS in the western population is 1 in 300; however, the worldwide prevalence may be substantially different (Wallace, 1971; Dogliotti et al, 1974; Jacyk and Isaac, 1979; Datta et al, 1993). The peak ages of recognition in women are bimodal, with many cases noted before puberty but with another peak presenting in postmenopausal women (Tasker and Wojnarowska, 2003). In men, LS seems to peak between the ages of 30 to 50; however, incidences of LS have been described in people of all ages, from infants to the elderly (Tasker and Wojnarowska, 2003). LS is commonly found at the time of circumcision when performed beyond the neonatal period (McKay et al, 1975; Rickwood et al, 1980; Garat et al, 1986; Ledwig and Weigland, 1989; Meuli et al, 1994). The most common cause of meatal stenosis, LS appears as a whitish plaque that may involve the prepuce, glans penis, urethral meatus, and fossa navicularis. If only the foreskin is involved, circumcision may be curative (Akporiaye et al, 1997). In the authors’ experience, LS usually begins as a meatal or perimeatal process in the circumcised patient, but it may involve other areas of the preputial space in the uncircumcised patient. In uncircumcised men, the prepuce becomes edematous and thickened, and often may be adherent to the glans (Bainbridge et al, 1971). Diagnosis is made through biopsy. Several reports have suggested the association with chronic infection by a spirochete, Borrelia burgdorferi (Tuffanelli, 1987; Dillon and Ghassan, 1995; Shelley et al, 1999). The term BXO was first applied by Stühmer in 1928. Freeman and Laymon showed that BXO and LS were probably the same process (Freeman and Laymon, 1941; Laymon and Freeman, 1944). The first report of what was probably LS was published by Weir in 1875. He described a case of vulvar and oral “ichthyosis” (Weir, 1875). In 1976, the International Society for the Study of Vulvar Disease unified the nomenclature devising a new classification system and proposed the term lichen sclerosus (Friedrich, 1976). As mentioned previously, the cause of LS has not been defined. A number of mechanisms have been proposed. Koebner phenomenon relates the development of LS to trauma to an affected area (Lee and Phillips, 1994). The etiology has also been suggested to be an autoimmune disease (Goolamali et al, 1974; Marren et al, 1995). Sander hypothesized that reactive oxidative stress contributed to the sclerotic, immunologic, and carcinogenic process in LS (Sander et al, 2004). As mentioned, the infectious cause has also been implicated (Tuffanelli, 1987; Ross et al, 1990). That has been called into question because the organism has not been uniformly demonstrated in the lesions. It has also been proposed that LS has a genetic origin, based on the observation of a familial distribution of cases (Marren et al, 1995). There have been reports of concomitant existence of the disease in identical twins (Fallic et al, 1997; Thomas and Kennedy, 1986) and nonidentical twins (Cox et al, 1986), with coexistence of dermatosis. The disease has also been seen in mothers and daughters (Shirer and Ray, 1987). Studies on the human leukocyte antigen (HLA) have suggested a genetic component in patients afflicted with LS (Marren et al, 1995). The combination of topical steroids and antibiotics may help stabilize the inflammatory process. Conservative therapy may be warranted in patients whose meatus can easily be maintained at 14 to 16 French (Staff, 1970). In these cases, intermittent catheterization with lubrication of the catheter and meatal dilator with 0.05% clobetasol (brand name Temovate) may be adequate treatment. Long-term antibiotic therapy may also be helpful to improve the inflammation, because secondary infection of the inflamed tissue may occur. We have typically used tetracycline, but a trial of long-term penicillin or advanced-generation erythromycin therapy may be warranted (Shelley et al, 1999). This nonsurgical approach to treatment is used in patients who are not good surgical candidates for other medical reasons or in older patients, and in younger patients who demonstrate stable disease. Secrest has proposed a link between hypogonadism and LS in male patients. He has consistently shown diminished testosterone levels in patients afflicted with LS and has a study underway analyzing whether replacement androgen therapy may be helpful (Secrest et al, 2008). In young patients with severe meatal stenosis, surgery is indicated. Because patients with long-standing meatal stenosis often have severe proximal urethral stricture disease, retrograde urethrography should be performed before the initiation of therapy. The etiology of stricture disease associated with LS is unclear. Possible causes include iatrogenic stricture resulting from repeated instrumentation and pressure voiding associated with meatal stenosis causing secondary intravasation of urine into the glans Littre (Fig. 36–14). In cases of early LS with only meatal involvement resulting in stenosis of the fossa navicularis, prompt reconstruction seems to be successful in the long term and seems to avoid the sequelae of panurethral stricture disease. Most surgeons now believe that because LS is a disease of genital skin, better tissue for reconstruction is the oral mucosa, and techniques are discussed later (Mundy, 1994; Bracka, 1999). Long-standing cases with a long length of urethral stricture are amenable to techniques of reconstruction but are very challenging. It is becoming clear that except in the case of urethral stricture disease confined only to the meatus and fossa navicularis, staged oral graft reconstruction, at least in the short to mid term, seems to provide superior durable results. This may also be the case in cases confined to the meatus and fossa navicularis, because a recent analysis of patients reconstructed with the ventral transverse skin island technique showed a 50% recurrence rate even in those patients. The weakness of that analysis, is that the data did not include biopsy proof that all patients had LS (Virasoro et al, 2007). We also are seeing more and more patients who present with a buried penis. This phenomenon occurs when the skin of the penile shaft has been lost because of severe inflammation, and the penis is trapped in the penopubic and scrotal area. These patients are often profoundly overweight, and many are diabetic; they have often had prior surgical procedures. Their management is complex and ultimately determined by their desire and need for functional reconstruction. In some patients with severe urethral stricture disease, we have completely reconstructed the urethra; in others, we have simply performed a perineal urethrostomy. Perineal urethrostomy is usually technically straightforward, because the rule in most patients with lichen sclerosus is to spare the proximal anterior urethra. We have proposed that, in many cases, the sparing of the proximal anterior urethra demonstrates the distribution of the glands of Littre for a given patient. Younger patients have requested mobilization and release of the penis with placement of a split-thickness skin graft. However, because the inflammation involves the glans penis (which is not removed), the secondary inflammation may also involve the skin graft. Therefore lifelong monitoring of these patients for the secondary effects of inflammation is necessary. (From Jordan GH. Management of membranous urethral strictures via the perineal approach. In: McAninch J, Carroll P, Jordan GH, editors. Traumatic and reconstructive urology. Philadelphia: WB Saunders; 1996.) Several reports have suggested the development of squamous cell carcinoma in patients with a long history of lichen sclerosus (Doré et al, 1990; Pride et al, 1993). Although a rare disease, amyloidosis of the urethra should be considered in the evaluation of any patient with a urethral mass. Patients may present with hematuria, dysuria, or urethral obstruction. Because the differential diagnosis includes urethral neoplasm, cystoscopy with transurethral biopsy is indicated. Once the diagnosis is made, treatment should be based only on symptoms. Most patients can be observed expectantly and not require aggressive treatment. Some will require treatment for urethral stricture. Progression and recurrence are rare (Walzer et al, 1983; Dounis et al, 1985; Crook et al, 2002).
Principles of Reconstructive Surgery
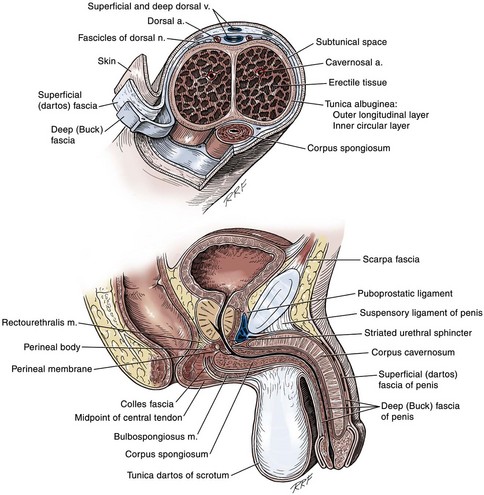
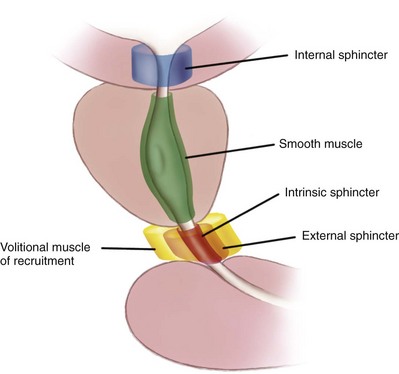
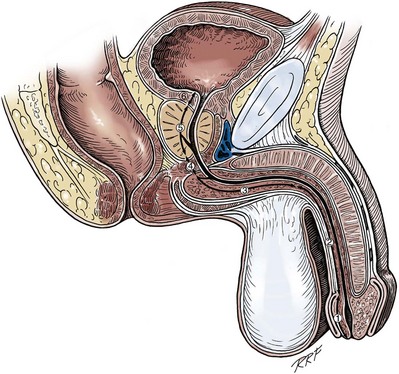
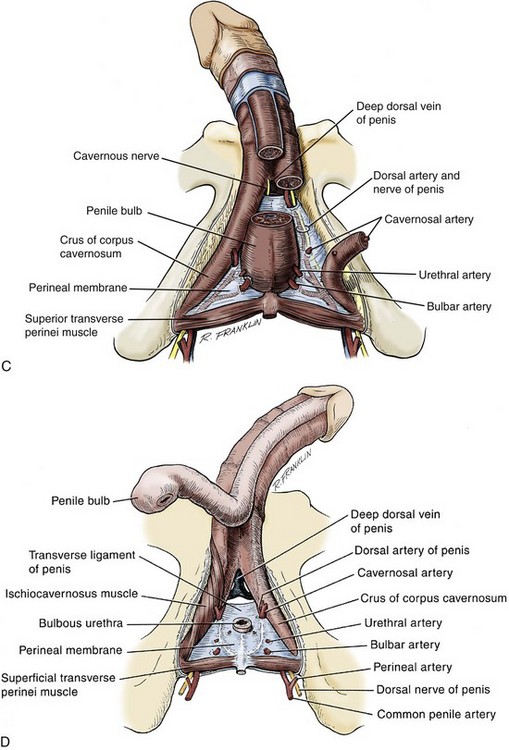
Anatomy of the Penis and Male Perineum
![]() .
.
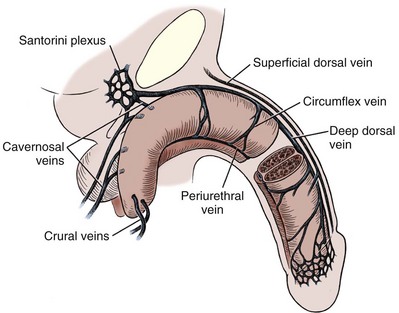
Venous Drainage
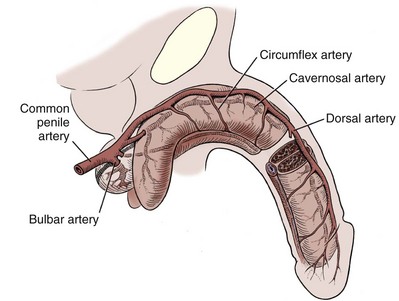
Arterial System
Perineum
Colles Fascia
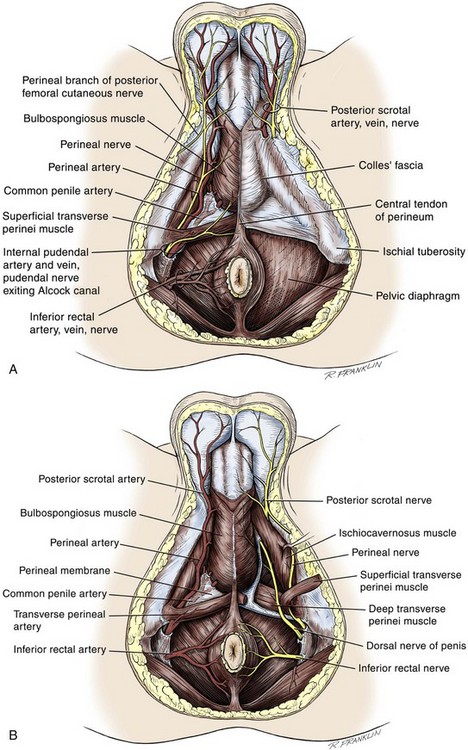
Superficial Perineal Space
Central Perineal Tendon (Perineal Body)
Deep Perineal Space
Generalities of Reconstructive Surgical Techniques
Selected Processes
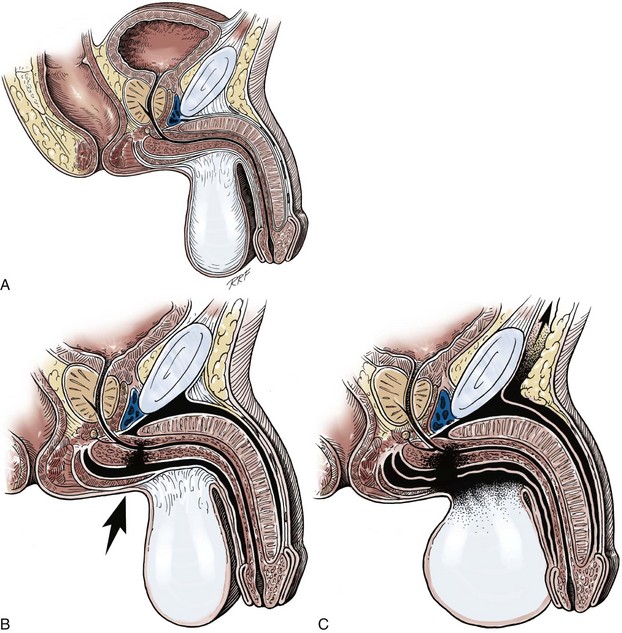
Lichen Sclerosus (Balanitis Xerotica Obliterans)
Amyloidosis
Surgery of the Penis and Urethra
• Many of the techniques in reconstructive surgery require the transfer of tissue. Tissue transfer implies the movement of tissue for purposes of reconstruction. All tissue has extensibility, inherent tension, and the viscoelastic properties of stress relaxation and creep. These physical characteristics are important in predicting the behavior of transferred tissue.
1. The fossa navicularis is contained within the spongy erectile tissue of the glans penis and terminates at the junction of the urethral epithelium with the skin of the glans. This portion of the urethra is lined with stratified squamous epithelium.
3. The bulbous urethra is covered by the midline fusion of the ischiocavernosus musculature and is invested by the bulbospongiosus of the corpus spongiosum. It becomes larger and lies closer to the dorsal aspect of the corpus spongiosum, exiting from its dorsal surface before the posterior attachment of the bulbospongiosus to the perineal body. The bulbous urethra is lined distally with squamous epithelium that gradually changes to the transitional epithelium found in the membranous urethra as it swings upward (Devine and Horton, 1977).
3. The prostate muscle continues into the membranous urethra as the external smooth muscle sphincter.
• Reconstructive surgery is performed with all efforts aimed at minimizing tissue injury and promoting healing. Loupe magnification is used by almost all surgeons performing adult and pediatric reconstructive surgery. For deep exposure, a headlight or lighted suction is advantageous. Instruments must be delicate because reconstructive surgery uses small sutures and small needles.