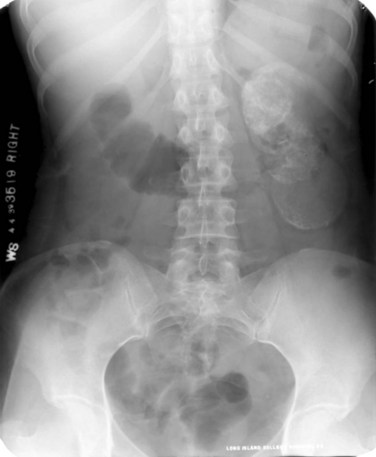
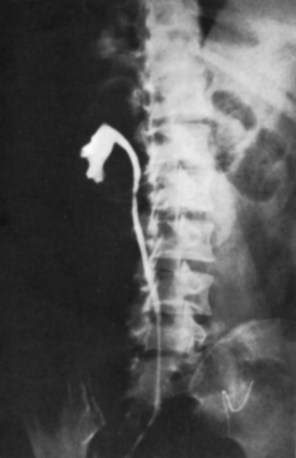
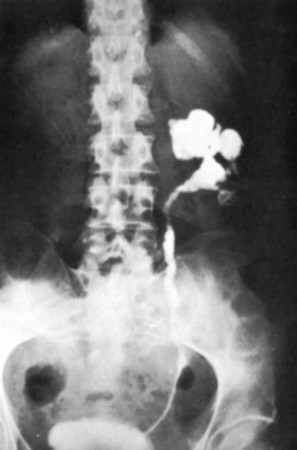
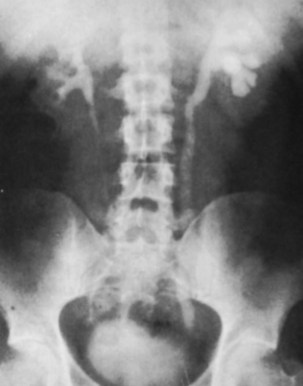
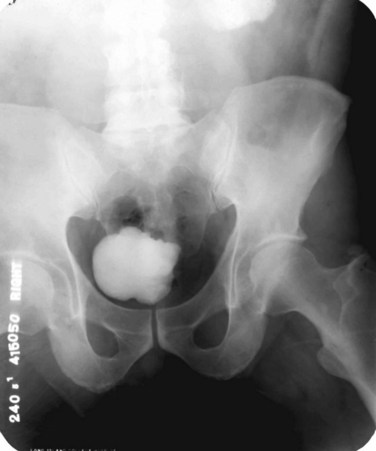
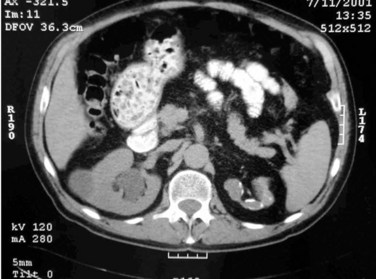
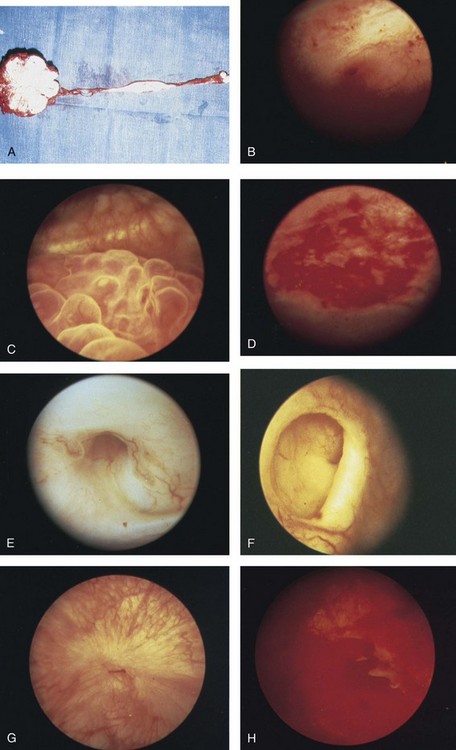
Islam A. Ghoneim, MD, PhD, John C. Rabets, MD, Steven D. Mawhorter, MD, DTM&H Akhenaton, a Pharaoh of the eighteenth dynasty of Egypt, and his wife Nefertiti both are believed to have died from tuberculosis, and evidence indicates that hospitals for tuberculosis existed in Egypt as early as 1500 BCE (Madkour, 2003). Signs of the disease have also been found in the spines of Egyptian mummies dating between 3000 and 2400 BCE (Zink et al, 2003). Tuberculosis became known as “the consumption” during the 1700s in Europe when infections reached epidemic proportions, causing one fourth of the deaths in England (Daniel, 2000). The bacillus causing tuberculosis, Mycobacterium tuberculosis, was identified and described on March 24, 1882 by Robert Koch. He demonstrated that Mycobacterium was the single cause of tuberculosis in all of its forms. His conclusion was based on the observations that Mycobacterium was found in all cases of the disease, could be prepared as a pure culture, and the original infection could be reproduced in an inoculated animal, from which it could be cultured again (Koch, 1882). These observations lead to postulates that have become known as the “Koch postulates,” and they form the basis for the study of all infectious diseases. March 24th has become “World Tuberculosis Day.” The World Health Organization (WHO) estimates that 9.27 million new cases of TB occurred in 2007, compared with 9.24 million new cases (140 per 100,000) in 2006. An estimated 1.37 million (14.8%) of the cases in 2007 were HIV-positive (World Health Organization and Global Tuberculosis Programme, 2008). Extrapulmonary sites account for 10% of tuberculosis cases. Genitourinary TB accounts for 30% to 40% of all extrapulmonary TB, second only to lymphonodal affection (Eastwood et al, 2001). In developed countries, urogenital tuberculosis occurs in 2% to 10% of cases of pulmonary tuberculosis, while in developing countries it occurs in as many as 15% to 20% of cases. Controversy existed as early as the latter part of the 19th century as to whether pulmonary tuberculosis spread to the genitourinary tract by bacterial retrograde ascent or hematogenous dissemination. In 1885, injection of the renal artery with TB bacillus was shown to produce renal tuberculosis in the kidney of an experimental animal. Ekehorn, in 1908, postulated that TB bacilli lodged in renal glomeruli flourished into renal infection. These suspicions were confirmed in 1949 by Medlar and associates, proving that renal cortical TB was a “metastatic” infection spread by the hematogenous route (Medlar et al, 1949). In the lung, inhaled tubercle bacilli implant in the respiratory bronchioles and alveoli. The interaction between bacterial virulence and host immunity determines whether an infection is established or aborted (Dannenberg, 1993). If infection occurs, the mycobacteria slowly divide within alveolar macrophages. Two to 12 weeks often ensue before mycobacterial numbers are sufficient to mount a clinically detectable cellular immune response (Dannenberg, 1994). In the presence of intact cell-mediated immunity, macrophages, T lymphocytes, B lymphocytes, and fibroblasts aggregate to form a granuloma, with lymphocytes surrounding the infected macrophages and organisms localized in the center of the granuloma. This pathognomonic lesion prevents dissemination of the mycobacteria. Immune cells communicate through cytokines within the milieu of the granuloma. T lymphocytes secrete interferon gamma, which induces intracellular killing of mycobacteria within infected macrophages (Kaufmann, 2002). An antibody response against M. tuberculosis has been demonstrated but does not appear to be protective (Abebe and Bjune, 2009). Tubercle bacilli often remain viable within the tubercle, become dormant, and finally result in a latent infection. The kidneys are the primary site of hematogenous spread of TB. Mycobacteria lodge in the renal capillaries causing microscopic foci near the glomeruli bilaterally, the cortex being favored due to its greater blood supply and higher oxygen tension (Pasternak, 2001). An initial acute inflammatory response ensues, resulting in polymorphonuclear leukocytes infiltration. Over the following 3 to 6 weeks cell-mediated immunity developing in macrophages may inhibit the M. tuberculosis by containing the bacterial replication and halting the disease in the renal cortex, leading to the formation of dormant TB foci. Upon activation of the disease, a chronic inflammatory process arises with the subsequent development of characteristic granulomata, tubercles consisting of multinucleated Langhans giant cells, lymphocytes, and fibroblasts. Central caseous necrosis builds up within the tubercles, and neighboring tuberculous foci coalesce to form confluent areas of caseation. With progression of the disease, inflammatory changes extend into the renal tubules and medulla with further tubercle formation and caseous necrosis. Renal papilla involvement results in sloughing and caseous material gaining access to the collecting system by calyceal ulceration (Medlar, 1926). Renal tuberculosis often becomes clinically evident at this stage. Extensive fibrosis accompanying healing tubercles results in cicatricial complications, such as calyceal infundibular narrowing, ureteropelvic junction scarring, and disfiguration leading to segmental or generalized hydronephrosis respectively, and adds an obstructive element to the ongoing renal damage (Medlar, 1926). Adrenal tuberculosis is seen in less than 6% of active TB cases. The lesion may be unilateral, but is usually bilateral. Tuberculosis causes necrosis of the adrenal gland, which manifests as Addison disease. Primary caseous tuberculosis of the adrenals is probably the most common lesion seen. The glands are enlarged, surrounded by a thickened capsule, and have irregular nodular surfaces with infrequent calcifications. Caseous cavitary destruction is found upon section. As a result, up to 56% of patients with adrenal TB will have a subnormal cortisol response to corticotrophin stimulation (Hawken et al, 1996). Tuberculosis of the ureter is almost always a direct extension of TB of the kidney. The passage of caseous material rich in mycobacteria leads to tubercle formation within the ureteric mucosa. This usually affects the lower ureter, commonly the ureterovesical junction, less commonly the middle and upper ureter (Shin et al, 2002). Tubercle formation is soon followed by ulceration of the mucosa and subsequent fibrosis and scarring, leading to ureteric stricture disease and obstruction. Lesions extending into the ureteric wall will also initiate a dense fibrosis on the ureteric serosa, leading to encasement of the ureter and angulation by contracted cicatricial bands (Johnson, 1911). Tuberculosis of the bladder occurs secondary to TB of the kidney. The bladder urothelium is very resistant to infection by TB bacilli. Urine of renal tuberculosis patients may contain mycobacteria for years prior to the involvement of the bladder. The most common sites affected by TB are the areas surrounding the ureteric orifices and the trigone. The urothelium is initially swollen and inflamed, following the formation of tubercles within the bladder mucosa. The ureteric orifice may be completely obscured by the swollen mucosa. In modern times the progression to the stage of mucosal ulceration is rare. However, if it does occur, the coalescence of tubercles will lead to larger areas of caseated mucosa, the top of which ulcerates leading to the formation of a classical undermined tuberculous ulcer with “worm-eaten” ragged edges (Johnson, 1911). The prostate is rarely affected, it is however one of the sites of hematogenous spread of TB. The lesions are often incidentally found on TUR specimens. In cases where progression occurs, caseous destruction of prostatic tissue ensues that may be significant enough to cause a noticeable reduction in semen volume (Marconi et al, 2009). Densely fibrotic nodules may form and are indistinguishable from cancer. One rare presentation is an ulcerative lesion of the glans. This primary infection can be acquired by sexual relations with partners having genital or perineal tuberculous lesions (Angus et al, 2001). Several cases of primary TB of the penis were reported in young Jewish boys following circumcision. Hemorrhage was stopped by sucking the penis with the mouth. Rabbis with open pulmonary TB transmitted the infection through infected sputum. Urethral tuberculosis is rare, often only seen at the meatus. Small miliary tubercles are seen over the surface and throughout the urethra. Late cases may have advanced fibrotic strictures. In the words of Chang: “the kidney is an inarticulate organ; its vocal cords are the bladder” (Chang, 1976). Tuberculosis can often mimic a wide range of nonspecific urologic symptoms. It is thus, no wonder that many cases of genitourinary TB are easily overlooked. A high index of clinical suspicion of TB is required to further investigate cases of unexplained symptoms in the urinary tract. This is especially important when there is a failure to respond to initial treatments given for lower urinary tract symptoms or when urinalysis and routine culture reveal “sterile pyuria.” The fact that 18 out of 25 physicians with renal TB presented only after cavitary lesions developed is a measure of how silently the destructive process occurs (Lattimer, 1965). Genitourinary TB is more commonly seen in men (male : female ratio of 2 : 1), usually presenting in the fourth decade of life. Lower urinary tract symptoms are the most common presentation, with over 50% of patients presenting with storage symptoms. Hematuria and loin pain are the presentation in one third of cases (Figueiredo et al, 2008). Passage of caseous material, necrotic renal papillary tissue, clots, or stones account for renal colic in 10% of patients (Simon et al, 1977). Less than 20% of cases will present with constitutional symptoms of fever, anorexia, weight loss, and night sweats. The presence of these symptoms, however, can alert to the presence of active TB elsewhere in the body (Simon et al, 1977). Physical examination is often of limited value in the diagnostic process, because physical signs develop late in the disease. The most common physical finding is an abnormal scrotal exam in about half the patients (Figueiredo et al, 2008). Epididymal hardening, modularity, or scrotal fistulae are among the signs seen. A chronic renal fistula tract, often with a history of prior renal surgery is another late physical sign. Enlarged, firm seminal vesicles, or prostatic nodules on rectal examination, though nonspecific, should arouse suspicion in clinically suggestive cases. It remains a fact that up to 25% of patients will present only with sterile pyuria and 13% might have gross or microscopic hematuria as their only presentation (Wise and Shteynshlyuger, 2008). Functional loss of the affected kidney can be present in up to 25% of cases, and renal failure is present in 7.4% of cases (Figueiredo et al, 2008). Genitourinary TB may be diagnosed during a workup for infertility as a cause of epididymal and vasal obstruction (Paick et al, 2000). TB should be considered in all cases of recurrent hemospermia. Adrenal tuberculosis may present with an addisonian type of clinical picture. Historically, the diagnosis of genitourinary TB has relied on the identification of Mycobacterium tuberculosis in the urine. Unlike sputum examination, Ziehl-Neelsen staining of concentrated urine samples for acid-fast bacilli is often negative. Of note, a large majority of patients with genitourinary TB have “sterile pyuria,” often accompanied by hematuria and proteinuria, whereas up to 20% may have superimposed bacterial infection (Gow and Barbosa, 1984). Urine cultures are carried out on standard solid media optimized for mycobacterial growth, namely egg-based (Löwenstein-Jensen) or agar-based (e.g., Middlebrook 7H10) media. Optimizing factors include aniline dyes, such as malachite green, that inhibit growth of bacterial contaminants. Agar-based media are transparent and facilitate earlier visualization of micro-colonies by approximately 1 week. Intermittent release of the organism in urine makes multiple sampling necessary. Three to five early-morning urine samples should be cultured soon after collection rather than 24-hour samples, because exposure to urine acidity for prolonged periods retards mycobacterial growth (American Thoracic Society [ATS] and Centers for Disease Control and Prevention [CDC], 2000; Sommers, 1979). One other caveat is that chronic renal lesions may no longer discharge tuberculous material in urine due to dense fibrosis that is a barrier to the collecting system. In such cases diagnostic methods other than urine testing must be applied. Notably, cultures optimized for mycobacterial growth will favor the growth of mycobacterial contaminants as well as TB. Species identification is done using growing colonies tested with DNA strip assays. These provide rapid confirmation of pathogenic Mycobacterium TB in culture (Piersimoni et al, 2002). Urine cultures are sensitive in 80% to 90% of cases and have a specificity of nearly 100% (Sorlozano et al, 2009). However, they may take up to 6 weeks to yield clinically reliable results. Radiometric detection of mycobacterial activity in liquid culture media allows a more rapid diagnosis. Inoculation of specimens in broth with radiolabelled 14C-palmitate results in liberation of 14CO2 by mycobacterial metabolism, which is then detected by a radiometric analyzer. Mycobacterial detection and drug sensitivity testing using this method is possible as early as 7 to 14 days after inoculation (Watterson and Drobniewski, 2000; Sorlozano et al, 2009). One of the most popular systems using this principle is the BACTEC 460TB (Becton, Dickinson, Franklin Lakes, NJ). Nonradiometric methods using advanced fluorometric technology to detect O2 consumption also yield rapid detection of mycobacteria without the use of radiation. One example of this detection method is the mycobacterial growth indicator tube (MGIT; Becton, Dickinson; Watterson and Drobniewski, 2000). Antibiotic sensitivity testing (AST) is also done using traditional and rapid culture methods. Charles Mantoux, a French physician who developed on the work of Koch, described this test in 1907. Purified protein derivative (PPD) tuberculin is a precipitate of non–species-specific molecules obtained from glycerol extracted filtrates of sterilized, concentrated cultures of tubercle bacilli. In the United States, a standard dose of 5 tuberculin units (0.1 mL) is injected intradermal (between the layers of dermis) into the volar or dorsal surface of the forearm and read 48 to 72 hours later. T-cell–mediated delayed-type hypersensitivity reaction to this intradermal antigen is the principle of the test. Antigen stimulation of memory cells leads to cytokine release that induces induration via local vasodilatation, fibrin deposition, and recruitment of other inflammatory cells into the area. These begin to accumulate within 24 hours and reach their peak after 48 to 72 hours, hence the timing of test interpretation. Patients who have been exposed to TB are expected to mount an immune response to PPD (Daniel, 1980). Tuberculin test is nondiagnostic, and is of value only if positive. Three distinct cut points for positivity have been defined to optimize sensitivity and specificity for the test in different patient populations. The test must be interpreted in light of the recommendations in Table 16–1. Table 16–1 Interpretation of Tuberculin Test: ATS and CDC Guidelines From American Thoracic Society and Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161(4 Pt. 1):1376–95. It is important to note that prior vaccination with bacillus Calmette-Guérin (BCG) may lead to a false-positive tuberculin test. Interestingly, a PPD skin test done before initiation of BCG therapy for superficial bladder cancer converted from negative to positive in 68% of patients (Bilen et al, 2003). Nucleic acid amplification tests (NAAT), such as the polymerase chain reaction (PCR) and other methods for amplifying DNA and RNA, facilitate rapid detection of microorganisms, particularly those difficult to culture. The high sensitivity of PCR is particularly useful in non-pulmonary tuberculosis where discharge of the organism is sporadic and present in small amounts (Manjunath et al, 1991). Multiple sampling is also necessary for this method. The PCR test has been extensively studied and has shown reliably high sensitivity, specificity, and rapid results. In various studies, data show sensitivity ranging from 87% to 95% (usually >90%) and specificity from 92% to 99.8% (usually >95%) as compared to culture. Staining for acid-fast bacilli, bladder biopsies, and intravenous pyelography (IVP) examinations all yielded inferior results (Hemal et al, 2000b; Moussa et al, 2000). Numerous commercial tests and kits are available with near-equivalent quality. Some caveats exist in the interpretation of NAAT results, because they are best used in conjunction with clinical judgment and TB cultures. Urine is known to have naturally occurring enzyme inhibitors in up to 10% of cases, which suppress the enzymatic reactions of DNA/RNA amplification (van Vollenhoven et al, 1996). This may result in false-negative tests (Moussa et al, 2000). It is thus important to corroborate with the laboratory that appropriate measures have been done to confirm a PCR result as negative rather than an inhibited reaction, especially in clinically suspicious cases. Moreover, NAA tests can amplify nucleic acids from dead organisms, thus yielding positive results even after effective chemotherapy. Thus they should be used for diagnosis only and not as a follow-up to treatment (ATS and CDC, 2000). Rapid molecular testing for drug resistance is available by detecting resistance mutations in three Mycobacterium TB genes. Results are available in 1 to 2 days (Barnard et al, 2008). Plain radiographic findings in genitourinary tuberculosis may be seen in the GU tract, surrounding tissues, and up to 50% of patients may show positive findings on chest radiograph. Disparity in renal size on plain films may indicate early increase in size of the affected kidney due to caseous lesions or a shrunken fibrotic kidney of autonephrectomy. Calcifications are seen in 30% to 50% of cases (Roylance et al, 1970). Focal calcifications occur within the caseating lesions (Fig. 16–1). A characteristic diffuse, uniform, extensive parenchymal, putty-like calcification, forming a lobar cast of the kidney is seen with autonephrectomy (Muttarak et al, 2005). Calculi may also be seen in the collecting system or ureter secondary to stricture formation. Ureteral calcifications are rare and are characteristically intraluminal as opposed to the mural calcifications of schistosomiasis. Bladder wall calcifications are not very common except in late cases of bladder contraction. Calcifications of the prostate and seminal vesicles are seen in 10% of cases (Burrill et al, 2007). Plain film findings suggestive of tuberculosis may be seen in surrounding tissues such as erosions of the vertebral bodies or calcifications in a cold abscess of the psoas muscle (Burrill et al, 2007). The majority of cases will show positive findings on excretory urography, the most common findings being hydrocalycosis, hydronephrosis, or hydroureter due to stricture formation (Wang et al, 2003). Early signs include the moth-eaten appearance of calyceal erosion and papillary irregularity. These signs are best seen on early excretory films, because they are often masked by increasing density of the contrast on later films of the IVU. Cavitary lesions communicating with the collecting system are characteristic of TB. These lesions eventually enlarge as parenchymal destruction ensues, and a picture similar to chronic pyelonephritis may be seen. Fibrotic distortion of the collecting system and ureter is also seen. Calyceal obliteration and amputation, hydrocalycosis, segmental or total hydronephrosis, and a shriveled reduced-capacity renal pelvis may all be signs of renal tuberculosis (Figs. 16-2 and 16-3). Scarring and angulation of the ureteropelvic junction (UPJ) may also occur, the so-called “Kerr’s kink” (Matos et al, 2005). Ultimately diminished or absent function and extensive calcification may be seen with autonephrectomy. If nonvisualized on IVU, the kidney is best evaluated by computed tomography (CT) or ultrasonography. Tuberculosis of the ureter is commonly seen as a rigid, straightened “pipe-stem” ureter. A beaded, corkscrew appearance is sometimes also seen. Ureterovesical junction obstruction is caused by tuberculous cystitis or strictures of the distal third of the ureter (Fig. 16–4). Secondary stone formation on top of this stricture is an occasional finding. The cystogram films may show a small contracted bladder due to excessive fibrosis (Fig. 16–5). Of note, although IVU is being phased out by CT-urography in many developed countries (Stacul et al, 2008), IVU continues to be a reliable imaging modality for genitourinary TB in most parts of the world. The most common findings on contrast-enhanced CT include renal parenchymal masses and scarring, thick urinary tract walls (ureter and bladder) and extraurinary tubercular manifestations particularly in miliary TB (Wang et al, 2003). Coalescence of caseating granulomata may lead to a renal mass (tuberculoma), which must be differentiated from renal cell carcinoma. CT allows for evaluation of renal function, grading of hydronephrosis and parenchymal scarring (Fig. 16–6). CT is most sensitive in detecting renal calcifications (Premkumar, et al, 1987). Most CT findings are in themselves nonspecific, and the collective interpretation of multiple findings in conjunction with the clinical picture is the best option in decision making (Wang et al, 2003). CT is also helpful in the diagnosis of adrenal tuberculosis, which may appear as bilaterally enlarged glands with areas of necrosis (caseation) early in the disease. Dotlike calcifications and atrophy of the adrenal gland are common late findings (Wang, et al, 1998). Tuberculosis of the prostate or seminal vesicles may lead to calcification, caseation, and necrosis causing hypoattenuation or cavity formation that can be visualized on contrast-enhanced CT scan of the pelvis. However, in the absence of calcification, tuberculous prostatic lesions may mimic a pyogenic abscess or carcinoma, especially that prostate-specific antigen (PSA) may be elevated in one third of cases (Lee et al, 2001). Needle biopsy may be needed in these cases. Endoscopy plays a limited role in the diagnosis of TB. Despite direct visualization of lesions, there are no pathognomonic findings that are specific for tuberculosis. Ulcerative lesions may mimic malignancy. A “golf-hole” ureteric orifice is suggestive of tuberculosis, and, when found, upper tract imaging or endoscopy should be obtained (Fig. 16–7). A positive urine culture or stain for acid-fast bacilli obviates the need for a biopsy, especially because it is diagnostic in only 18% to 45% of cases (Wong et al, 1984; Hemal et al, 2000b). However, a biopsy should be done when in doubt of malignancy. Successful treatment of genitourinary tuberculosis relies on the early diagnosis and the prompt initiation of an adequate drug regimen. Prior to successful antituberculous chemotherapy extirpative surgery was the initial form of treatment. Currently surgical treatment is reserved for advanced cases, often to correct the obstructive effects of fibrosis and scarring rather than the removal of infected tissues. Thus the balanced medical-surgical approach is ideally aimed at the preservation of renal (organ) function and eradication of mycobacteria. Despite standardized regimens and surgical indications, treatment must also be individualized; “No disease process is as atypical as a typical tuberculous process” (Cooper and Robinson, 1972). The principle underlying medical treatment is the effective eradication of the slowly dividing mycobacteria from tissues and urine. To understand the basis for modern-day multidrug regimens for tuberculosis, it is important to contemplate the following dilemma: Mycobacteria exist in several different environments in genitourinary tract TB. The largest population is the more actively dividing extracellular mycobacteria that exist within cavitary lesions, often at a neutral or alkaline pH. Another population exists in the intracellular acidic environment within macrophages. A smaller population of slowly dividing organisms can be found enclosed within caseous material at a neutral pH or freely in the acidic pH of urine (Dutt and Stead, 1982). The differential ability of various antimycobacterial drugs to penetrate tissues, inherent effects on the tubercle bacillus (bactericidal vs. bacteriostatic), and their levels of activity in the wide range of pH required to manage genitourinary TB (as discussed under the individual drugs below), dictates the use of a multidrug regimen. This is in addition to the standard principle of lowering the emergence of drug-resistant strains. Genitourinary TB can safely be managed with short-course chemotherapy (Dutt et al, 1986; Small and Fujiwara, 2001). This treatment is effective because there are fewer organisms in genitourinary lesions; isonicotinic acid hydrazide (isoniazid, INH) and rifampicin have good penetration of lesions at lethal concentrations; and isoniazid, rifampicin, and pyrazinamide attain high concentrations in urine (Gow and Barbosa, 1984). Classically, antimycobacterial regimens rely on a first line of drugs, namely, rifampicin, INH, pyrazinamide, and ethambutol. A second line of drugs is reserved for cases that fail to respond to first-line therapy or in drug-resistant cases. Finally, it is important to acknowledge the rising incidence of multidrug resistant (MDR-TB) and extensively drug resistant TB (XDRTB) infections, and the resultant implications for the drug regimens used (Johnston et al, 2009). A standard treatment regimen for tuberculosis requires 6 months of therapy. The first 2 months involve three to four drugs: Rifampicin, isoniazid, and pyrazinamide are administered daily; ethambutol is added if drug resistance to isoniazid is suspected. An additional 4 months of rifampicin and isoniazid daily, twice per week, or three times per week are used (Small and Fujiwara, 2001). The intensive 2-month, three-drug regimen targets rapid multipliers, and the prolonged 4-month, two-drug regimen eradicates slow, sporadic multipliers and persistent bacteria. Directly observed therapy is often best to ensure patient compliance and completion of treatment. It is important to obtain adequate specimens for culture and susceptibility testing before treatment is initiated. Ethambutol can be stopped if lack of resistance is demonstrated. Baseline measurements of hepatic enzymes, bilirubin, and creatinine, and a complete blood count with a platelet count, should be performed. During treatment, all patients should be monitored for adverse effects on a monthly basis. Routine follow-up of liver enzymes is important to detect hepatotoxicity of rifampicin. If pyrazinamide is used, uric acid levels should be measured, and if ethambutol is given, visual acuity and red-green color perception should be monitored (Small and Fujiwara, 2001) (Tables 16-2 and 16-3). Table 16–3 Some Second-Line Antituberculous Drugs * Streptomycin is generally given 5 to 7 times per week (15 mg/kg, or a maximum of 1 g per dose) for an initial 2- to 12-week period and then, if needed, two to three times per week (20 to 30 mg/kg, or a maximum of 1.5 g per dose). For patients >59 years old, dosage is reduced to 10 mg/kg/day (max 750 mg/day). Dosage should be decreased if renal function is diminished. † Some authorities recommend pyridoxine, 50 mg, for every 250 mg of cycloserine to decrease the incidence of adverse neurologic effects. From The Medical Letter. Drugs for tuberculosis. Treat Guidel Med Lett 2009;7(86):75–82; quiz 2 (after p. 82). Isoniazid (INH) acts by inhibition of cell wall lipid synthesis, depletion of nucleic acid pools, and metabolic depression in mycobacteria (Timmins and Deretic, 2006). It penetrates caseous material and is active within the macrophages. INH is rapidly and completely absorbed orally; this may be slowed by food. It is widely distributed in the body, and tissue levels are similar to serum levels. Seventy percent of all administered INH is excreted by the kidneys, most in an inactive form. Dose modification in renal failure is usually not necessary but is recommended in hepatic failure, especially in persons who are slow hepatic acetylators. Rifampicin acts by inhibiting the β-subunit–dependent DNA-directed RNA polymerase of mycobacteria, leading to suppression of DNA synthesis (Sousa et al, 2008). The drug is lipid soluble, enters macrophages, and is excreted in the urine. Extensively drug-resistant tuberculosis (XDR-TB) refers to M. tuberculosis that is resistant to at least isoniazid and rifampin, and is additionally resistant to fluoroquinolones and either aminoglycosides (amikacin, kanamycin) or capreomycin, or both (Centers for Disease Control and Prevention [CDC], 2007). Typical MDR-tuberculosis regimens can consist of up to five drugs, used for a minimum of 18 months after culture conversion to negative. XDR tuberculosis requires individualized treatment based on drug-susceptibility testing of the particular organism (Rich and World Health Organization, 2006). Drug regimens combining linezolid with other companion drugs selected according to individual drug history, and tailored to the susceptibility results of each isolate, are currently being evaluated (Schecter et al, 2010). About 55% of patients with genitourinary TB will require surgical intervention. This rate is lower in areas where the disease is diagnosed early, while still asymptomatic. In developing countries, where the disease is diagnosed in late stages, often after examination of nephrectomy specimens, this rate maybe as high as 95% (Figueiredo et al, 2008). The role of surgery has changed in the era of effective antitubercular treatment. Surgical intervention compliments medical treatment in preservation and restoration of organ function. Ablative surgery is performed less commonly and should be considered carefully. The earlier diagnosis and chemotherapy have allowed reconstructive procedures to be performed more commonly today, even in advanced cases. Currently, more than half of surgeries performed for TB are reconstructive (Gupta et al, 2006). Moreover, surgical treatment is best carried out after an initial 3 to 6 weeks of medical treatment. This interval allows intense inflammatory changes to resolve and lesions to stabilize, permitting a better assessment of the extent of destruction, and hence doing the appropriate procedure. In the setting of obstruction and deteriorated kidney function, initiation of medical treatment can temporize definitive surgical intervention until renal function recovers, provided that measures are taken to relieve the obstruction (e.g., ureteric stenting or percutaneous nephrostomy). The prompt relief of obstruction is emergently required in cases of uremia or sepsis. Bilateral hydronephrosis or unilateral hydronephrosis of a solitary or functionally solitary kidney is often the cause of renal failure. Early ureteral stenting or percutaneous nephrostomy (PCN) for tuberculous ureteral strictures have been demonstrated to decrease the loss of renal function and increase the opportunity for later reconstructive surgery (Shin et al, 2002). In such cases, temporary and immediate drainage of hydronephrosis, preferably by retrograde ureteric stenting if possible, is required. An indwelling double J stent can be left until the patient’s condition is optimized. Retrograde placement is successful in 41% of cases (Ramanathan et al, 1998). When this is not technically feasible, percutaneous puncture of the hydronephrotic kidney is done to pass an antegrade stent. If that also fails, a percutaneous tube is left until definitive management is done. In cases of segmental hydronephrosis, more than one PCN may be required to achieve adequate drainage (Carl and Stark, 1997). It is important to understand that PCN placement must be followed by correcting the cause of obstruction. A tuberculous cutaneous fistula invariably develops if the PCN is simply removed. If the renal unit is deemed unsalvageable or shows no function, a nephrectomy is inevitable to prevent this complication. One pitfall to avoid during stent placement is the use of high-contrast injection pressures, because this may lead to possible dissemination of infection (Salem, 2008). Upfront (primary) nephrectomy to remove an infected kidney or debride caseous tissue is no longer the preferred line of treatment of genitourinary TB in the era of modern antitubercular chemotherapy. Today, the decision for a nephrectomy is based upon the extent of renal parenchymal destruction and, more importantly, the function of the kidney (Ramanathan et al, 1998). The indications for nephrectomy have thus been reduced to the excision of a renal unit that is nonfunctioning despite adequate drainage and medical treatment (Gupta et al, 2006). Other indications include extensive parenchymal destruction involving the whole kidney associated with hypertension (Flechner and Gow, 1980). Coexisting renal carcinoma also mandates nephrectomy. The current paradigm implies that chemotherapy is sufficient to render lesions free of mycobacteria even in nonfunctioning kidneys. However, removal of a calcified nonfunctioning kidney may be curative in patients with persistent symptoms (predominantly those of cystitis), because up to 50% of cases may still discharge mycobacteria in urine despite adequate chemotherapy (Fischer and Flamm, 1990). A standard lumbar incision provides adequate exposure sufficient to carry out the dissection and control of the kidney. Every effort should be taken to avoid inadvertent entry into the peritoneal or pleural cavities. It is often possible to preserve the adrenal gland. In rare cases, the perinephric fat may appear to have tubercles or caseous cavities. These should not be dissected from the kidney and should be removed with the specimen. Dense fibrosis, due to healing of such lesions after chemotherapy, is more commonly encountered in the perirenal tissues or surrounding the renal pedicle. Control of the renal artery and vein, separately, may be difficult in these cases, and the pedicle may be controlled in toto by suture ligation. Routine removal of the ureter is not necessary. Retroperitoneoscopic nephrectomy has been attempted successfully for tuberculous kidneys (Hemal et al, 2000a). Strictures forming during medical treatment and managed by early stenting (double J placement) have been shown to stabilize and require no further treatment (Shin et al, 2002). Balloon dilatation by retrograde or antegrade access has been described for TB strictures of the ureter, UPJ, and calyceal infundibula (Murphy et al, 1982; Kim et al, 1993). A stent is left after dilatation. Due to high failure rates, repeated procedures are needed. Follow-up of all cases of ureteric strictures by imaging (ultrasonography or IVU), especially those managed endoscopically, is needed because some strictures will worsen during the healing process due to fibrosis and cicatrization. Corticosteroids may be added if deterioration is detected. Consequently, up to 72% of cases will show relief of obstruction (Horne and Tulloch, 1975), and stent placement, if still needed, is often possible at this time. Failure to respond or progression after 6 weeks is an indication for definitive management. Repair of the ureteropelvic junction scarring is more challenging in TB cases than for congenital stenosis. The choice of procedure largely depends on the degree of scarring and contraction of the pelvis. Dismembered pyeloplasty is feasible for extrarenal pelvis with short segment scarring. Nondismembered (flap) pyeloplasty is preferred for longer strictures, but may not be feasible due to excessive scarring of the pelvis. When anatomic reconstruction is not possible, ureterocalicostomy (ureter to the lower pole calyx) is an option. It is important to cover the exposed lower pole parenchyma (using preserved capsule or omentum) to avoid fibrosis and constriction around the anastomosis (Carl and Stark, 1997). Augmentation cystoplasty and bladder substitution are options in the management of the tuberculous contracted bladder. A capacity of less than 100 mL is commonly the indication to augment. Extremely contracted bladders (thimble bladders of 20 mL capacity) are best managed by orthotopic bladder substitution (Hemal and Aron, 1999). Various bowel segments have been used, and the general rules of incorporating the bowel into the urinary tract apply, such as thoroughly evaluating renal functions, reconfiguring a low pressure reservoir (de Figueiredo et al, 2006), patient education, and long-term follow-up.
Genitourinary Tuberculosis
History
Epidemiology
Incidence
Genitourinary TB
Transmission and Development of Disease
Immunology and Pathogenesis
Pathologic Features
Kidney
Adrenal
Ureter
Bladder
Prostate and Seminal Vesicles
Penis and Urethra
Clinical Manifestations
Diagnosis
Urinalysis and Culture
Purified Protein Derivative–Tuberculin Test—Mantoux Test
Nucleic Acid Amplification (NAA) Testing—PCR
Radiography and Endoscopy
Plain Radiograph
Intravenous Urography (IVU)
Computed Tomography
Cystoscopy and Ureteroscopy
Treatment
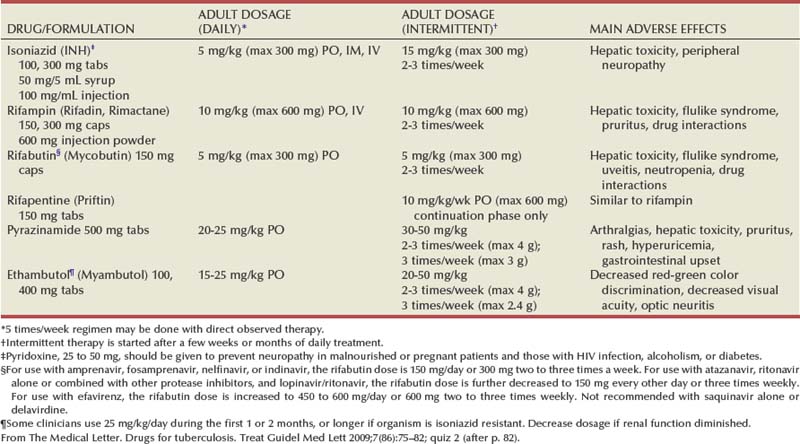
Medical Therapy
DRUG/FORMULATION
ADULT DOSAGE
(DAILY)
MAIN ADVERSE EFFECTS
Streptomycin*
15 mg/kg IM, IV (max 1 g)
Vestibular and auditory toxicity, renal damage
Capreomycin (Capastat)
15 mg/kg IM, IV (max 1 g)
Auditory and vestibular toxicity, renal damage, electrolyte imbalance
Kanamycin (Kantrex and others)
15 mg/kg IM, IV (max 1 g)
Auditory toxicity, renal damage
Amikacin (Amikin)
15 mg/kg IM, IV (max 1 g)
Auditory toxicity, renal damage
Cycloserine† (Seromycin and others)
10-15 mg/kg in two doses (max 500 mg bid) PO
Psychiatric symptoms, seizures
Ethionamide (Trecator-SC)
15-20 mg/kg in two doses (max 500 mg bid) PO
Gastrointestinal and hepatic toxicity, hypothyroidism
Levofloxacin (Levaquin)
500-1000 mg PO, IV
Nausea, abdominal pain, restlessness, confusion, rash dysglycemia
Moxifloxacin (Avelox)
400 mg PO, IV
Nausea, abdominal pain, restlessness, confusion, rash dysglycemia
Aminosalicylic acid (PAS; Paser)
8-12 g in 2-3 doses PO
Gastrointestinal disturbance
Antituberculous Drugs
Isoniazid
Rifampicin
Multidrug-Resistant TB
Surgical Therapy
Procedures to Relieve Obstruction
Nephrectomy
Ureteropelvic and Ureteral Surgery
Endoscopic Management
Surgical Options
Bladder Surgery
Tuberculosis and Other Opportunistic Infections of the Genitourinary System