Harry W. Herr, MD
According to the American Cancer Society, cancers of the urinary bladder accounted for an estimated 70,980 new cases and 14,330 deaths in the United States in 2009 ( Jemal et al, 2009 ). These cancers are the sixth leading cause of cancer death among Americans, and they are a significant source of health care costs.
Surgery of bladder cancer consists of transurethral resection for diagnosis and cure of noninvasive or minimally invasive neoplasms and radical cystectomy and pelvic lymph node dissection for invasive cancers. Radical cystectomy for transitional cell carcinoma (TCC) of the bladder serves as the gold standard for local control and survival of muscle-invasive tumors. The aim of the procedure is to remove all cancer in the bladder, pelvis, and regional lymph nodes with a wide soft tissue margin. The plane of dissection is the musculoskeletal boundaries of the pelvis.
Early attempts at cystectomy met with poor survival outcomes. In 1887 Bardenheuer of Cologne, Germany performed the first cystectomy. The procedure was one in which only the bladder was removed and neither ureter was diverted. The patient died a few days after surgery from sepsis, and other contemporary attempts often met with a similar outcome.
The first publication of long-term outcomes using the techniques of radical surgery with wide local excision and removal of regional lymph nodes with urinary diversion came from Whitmore and Marshall (1962) . In their landmark publication, they observed 5-year survival rates of 21% to 49% in their series of 230 patients. Since then, advances in endoscopic and open surgical technique, as well as perioperative care, have reduced the surgical mortality from more than 10% to less than 5% and improved results. Additionally, the use of neoadjuvant chemotherapy treatment before surgery has led to increased long-term survival. Recent large series have shown 10-year cancer-specific survival rates in patients with pathologic T2 cancers of 65% to 78% (Stein et al, 2001 ; Shariat et al, 2006 ).
Since the introduction of the cystoscope by Nitze in 1877, endoscopic diagnosis and treatment have been the initial steps in the management of bladder tumors. Flexible office cystoscopy and sometimes biopsy are performed to establish presence of a bladder tumor in patients presenting with gross hematuria, persistent microscopic hematuria, or irritative voiding symptoms such as dysuria. An examination under anesthesia and endoscopic resection are performed under spinal or general anesthesia to remove the tumor completely, if possible, and to obtain sufficient pathologic material for staging. In the case of noninvasive (superficial) disease, and in some cases muscle-invasive disease, resection alone can be curative.
Initial presentation to the urologist is often through a referral for microscopic or macroscopic hematuria, although other presenting symptoms can include irritative voiding, bladder outlet obstruction, abdominal mass, or flank pain. Occasionally, patients present with a tumor found incidentally either through imaging or an unrelated cystoscopy. At presentation, a history and physical examination are performed, with special attention paid to environmental exposures. Current or past tobacco use, exposure to aromatic amines in the rubber and dye industry, cyclophosphamide-based chemotherapy, and radiation exposure (particularly for prostate cancer) are all known risk factors for developing bladder cancer. Physical examination includes palpation of lymph nodes, flank, and abdomen. Men undergo a digital rectal examination to assess possible prostate involvement, and women have a bimanual examination to palpate size, location, and extent of the bladder tumor.
Laboratory studies comprise a complete blood count including white blood cell count, hemoglobin, hematocrit, and platelet count because many patients have underlying infection and anemia. A basic metabolic panel including serum electrolytes and creatinine with a calculated glomerular filtration rate demonstrate any underlying metabolic or renal dysfunction. Prothrombin time (with calculated international normalized ratio) and partial thromboplastin time are included as well, with any abnormalities addressed preoperatively. A urinalysis and urine culture should be sent before resection because many patients are colonized with bacteria and yeast before resection and require appropriate preoperative antibiotic treatment or prophylaxis.
Before the endoscopic treatment of a bladder tumor, the patient should undergo upper tract imaging to rule out the presence of an upper tract TCC. For patients with a glomerular filtration rate greater than 60 mL/min and no allergies to intravenous (IV) iodinated contrast, the standard modality in most centers is a computed tomography (CT) urogram, which can combine abdominal imaging for local spread or metastasis with delayed images that can detect intraluminal defects of the upper tract. Where unavailable, intravenous pyelography is a reasonable alternative for the detection of intraluminal filling defects. For a patient with a glomerular filtration rate (GFR) of 30 to 60 mL/min or an IV contrast allergy, magnetic resonance urography provides a safer alternative to CT urogram and provides the combined benefits of abdominal imaging with effective upper tract imaging. For patients with chronic kidney disease and a GFR less than 30 mL/min, upper tract imaging should include renal ultrasound and a retrograde pyelogram to image the ureter and renal pelvis.
In the operating room, the patient is placed in the dorsal lithotomy position after a local or general anesthetic induction. An appropriate antibiotic is given within 1 hour of initiation of the procedure, with either a second-generation cephalosporin or a quinolone given in the event of a preoperatively negative urine culture. Initially a bimanual examination of the pelvis is performed to determine clinical staging of the tumor. Then a complete endoscopic evaluation of the urethra, prostate (in males), and bladder are performed using both a 30- and 70-degree angled lens. Both ureteral orifices are examined for proximity to a tumor and involvement in the disease process. The bladder is filled with either water or 1.5% glycine irrigant (in the case of monopolar electrocautery being used) or 0.9% sodium chloride solution (if bipolar electrocautery is used).
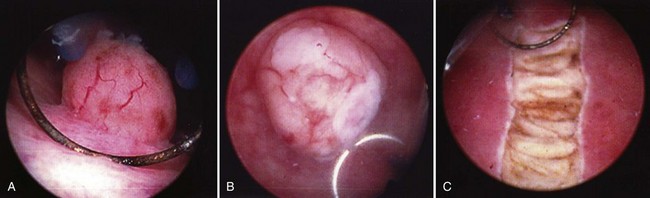
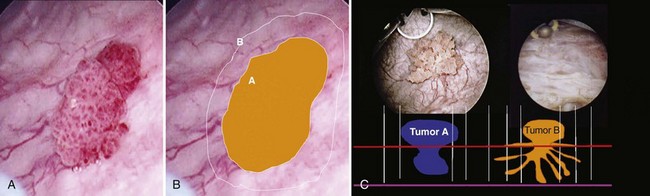
Once the tumor or tumors are identified, resection is performed using a cutting current on the loop resection device down to the level of the detrusor muscle. It is essential to resect to this depth to obtain sufficient tissue for pathologic staging and for completeness of resection ( Fig. 83–1 ). The margin of resection should include a 2- to 3-cm visually negative margin as deep muscle spread of tumor can occur in either a broad-based or tentacular fashion (Fig. 83–2 ). Resection of tumors involving the lateral bladder wall risk stimulation of the obturator nerves, leading to rapid thigh adduction and possible perforation of the bladder. This can be minimized by reducing the cautery current and rapidly tapping the foot pedal. Smaller and multiple lesions can be treated with cold cup biopsy and fulguration or fulguration alone on a coagulation current, or with another energy source such as a neodynium-yttrium-aluminum garnet laser (Nd : YAG).
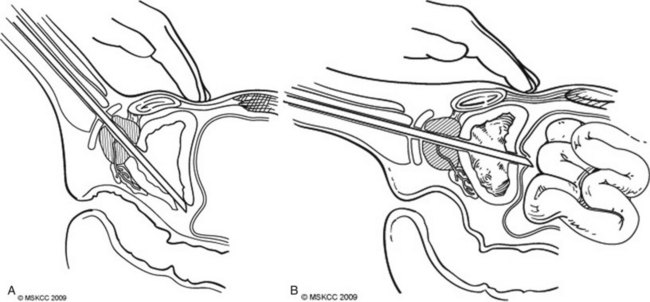
Bladder perforation is a complication that occurs in resections carried to the optimal depth of resection into the detrusor muscle. On the basis of the location of the perforation, these result in either an extravesical or intravesical extravasation of irrigant. Perforations that occur at the trigone, bladder base, and laterally typically result in an extravesical perforation with irrigant extravasation and can often be managed conservatively with Foley catheter drainage (Fig. 83–3A ). Perforations at the bladder dome can result in intraperitoneal perforation and extravasation and often require operative repair and drainage of irrigant (Fig. 83–3B ). Intraperitoneal ruptures are often diagnosed intraoperatively with palpation of the abdomen confirming distension from irrigant. An intraoperative cystogram can confirm rupture of either type.
Following resection without bladder perforation, instillation of intravesical chemotherapy with agents such as doxorubicin or mitomycin-c can modestly reduce recurrence rates from 15% to 38% but have little effect on progression (Kurth et al, 1997 ; Huncharek et al, 2001 ). Instillation of BCG, which is reserved for superficial high-grade disease, has shown superior efficacy in both recurrence rates and progression but should be instilled 2 to 4 weeks after resection due to the potential risks of “BCG-osis” with a disrupted urothelium (Lamm et al, 1991 ).
Surgery on the bladder first involves preoperative evaluation with staging through CT scan of the chest, abdomen, and pelvis to rule out metastatic spread of disease. Patients should be medically optimized before surgery because many of the patients have preexisting comorbidities such as chronic obstructive pulmonary disease, coronary artery disease, and anemia. Also, the site of a diverting urinary stoma should be marked with an enterostomal therapist in the preoperative setting to locate an appropriate placement for the patient in a standing and seated position. Of note, even in patients who have selected to undergo orthotopic neobladder construction, a stomal site should be selected in the event that a neobladder cannot be performed.
The patient should have a clear liquid diet the day before surgery and nothing by mouth after midnight. A mechanical bowel prep with 4 L of polyethylene glycol 3350 and electrolytes should be administered starting at 12 PM the day before surgery. Many patients are dehydrated the morning of surgery, and intravenous hydration with iso-osmolar crystalloid should be initiated. Intravenous antibiotics should be initiated within 1 hour of incision with a third-generation cephalosporin and metronidazole.
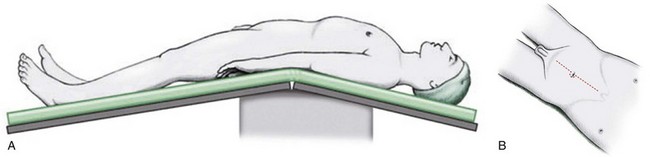
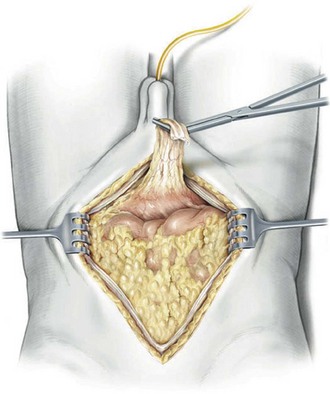
The patient is positioned in the supine position with the table flexed at the level of the anterior superior iliac spine of 15 degrees ( Fig. 83–4 ). Spreader bars or low-lying stirrups are helpful, especially to facilitate access to the perineum in males or vaginal vault in females. The abdomen is prepared with a 10% povidone-iodine solution from below the xiphoid process to the upper thighs, with the perineum and inner thigh prepped as well. A vaginal prep should be done in the female patient. An incision is made in the midline from 2 cm above the umbilicus to the pubis, although in many cases the operation can be performed only through an infraumbilical incision, facilitating a faster recovery. The underlying anterior and posterior layers of the fascia are divided, and the extraperitoneal space is entered. The bladder is then mobilized to the pelvic side walls on both sides. Once this space is developed, a peritoneotomy is made and the peritoneum is entered. The urachal remnant is then encircled and divided near the level of the umbilicus (Fig. 83–5 ). The peritoneum is incised lateral to the medial umbilical ligaments to the internal inguinal rings bilaterally. The peritoneum overlying the bladder should always be removed with the specimen. The vas deferens is encountered and divided.
At this time, the root of the small bowel mesentery is mobilized from the right lower quadrant to ensure appropriate exposure of the right ureter, gonadal vessels, and great vessels in preparation of the lymph node dissection, while the descending colon is mobilized laterally at the white line of Toldt ( Fig. 83–6 ). A large peritoneal window is developed with the sigmoid colon to allow a sufficient gap through which the left ureter can pass for the urinary diversion.
Only gold members can continue reading.
Log In or
Register to continue
Stay updated, free articles. Join our Telegram channel
Join
Full access? Get Clinical Tree
![]()