John M. Fitzpatrick, MCh, FRCSI, FC Urol (SA), FRCSGlas, FRCS
Intraprostatic Stents
One of the earliest attempts to find less traumatic methods of treating symptomatic BPH was the introduction of either temporary or permanent intraprostatic stents, and, to a degree, they are still being used. They were first introduced as a method of treating certain cardiovascular conditions; and since the work of Dotter (1969), they have also been part of the treatment for peripheral vascular disease. They have since been used to treat stenosis of the coronary, femoral, and renal arteries (Maass et al, 1982; Dotter et al, 1983; Wright et al, 1985; Palmaz et al, 1987). Other types of obstruction have also been treated by the placement of stents: vena caval obstruction (Furui et al, 1990), bronchial obstruction (Mair et al, 1990), and tracheal stenosis (Skapshay et al, 1989). Even cervical stenosis (Luesley et al, 1990) and lacrimal duct obstruction (Hurwitz, 1989) have been treated in this manner. It is clear that stents have a role to play in treating obstruction in different parts of the body, but the exact role needs to be defined accurately. In the case of coronary artery disease, for example, a trial comparing stents with coronary artery bypass grafting is still awaited. In the case of the prostate, it took some time before it was realized that the role of stents was rather a limited one and that they would not replace TURP in every patient.
The idea of using stents for splinting the lobes of the prostate was derived from their original use in the cardiovascular system, where they are used to prevent arterial restenosis after angioplasty. Fabian (1980) first described the use of stents in urology when he suggested their usefulness in the treatment of outlet obstruction secondary to enlargement of the prostate. At some time after this the use of stents was advocated in the treatment of urethral strictures, and, subsequent to this, the use of prostatic stents became widespread, with the introduction of many different types: stents are now available in different lengths, diameters, materials, and designs.
Temporary Stents
Spiral Stents
First-Generation Stents
A spiral stent should be inserted with a 21-Fr panendoscope using either a 30-degree or a 0-degree lens under direct vision and with the aid of a grasping forceps. It may also be inserted over a catheter guide under ultrasound visualization (Nordling et al, 1989).
In the original series reported by Nordling and colleagues (1989), the Prosta Kath was inserted under topical anesthesia in 45 patients with acute or chronic retention. Ultrasonography was used in 35 patients and endoscopy in 6 to facilitate stent placement. Retention was relieved in 41 of the 45 patients, leading the authors to advocate its use in high-risk patients.
Ozgur and coworkers (1993) reported re-establishment of voiding with the Urospiral with good results after 4 months of follow-up in 31 patients who were unfit for surgery. The Urospiral was also used in 10 patients with advanced prostate cancer (Anson et al, 1993). All had retention or severe obstruction. The patients were started on antiandrogens after stent insertion. The stent was removed 3 months later, and all patients were reported as voiding satisfactorily, although one patient subsequently required a limited TURP. In another report, 18 high-risk patients with BPH had a Urospiral inserted (Karaoglau et al, 1992). All voided without difficulty and with complete bladder emptying. However, the complications were rather high: hematuria in 2, migration in 1, and infection in 8.
In 87 patients declared unfit for TURP, Thomas and colleagues (1993) reported an experience extending over 4 years using the Prosta Kath. Sixty-four patients presented with acute urinary retention, and, after treatment, 57 voided successfully whereas 7 failed to void. A further 14 patients presented with chronic urinary retention; 5 of these voided satisfactorily, but 9 required alternative therapy. Complications included hematuria with clot retention (5%), stent migration (15%), recurrent urinary tract infections (10%), and encrustation (4%). These findings of relatively high complications are also reported by other authors (Nordling et al, 1989; Harrison and De Souza, 1990). Braf and coworkers (1996) observed 55 men for between 12 and 16 months, 32 of whom were treated with the Prosta Kath and 23 with the Urospiral. Ten patients failed in the Prosta Kath group, and 8 patients failed in the Urospiral group. Complications in both groups include encrustation, urinary tract infection, migration, stricture formation, and failure to void. In the largest number of patients reported from one center Nordling and associates reported on the use of the Prosta Kath in 318 patients. They divided the complications into none, moderate, and severe. In the patients who were described as having severe complications, stress or urgency incontinence occurred in 63, emptying problems in 8, and frequency or nocturia (more than three times per night) or both in 57.
Second-Generation Stents
The Memokath was reported as being used in 30 patients who were unfit for surgery or who refused it; the success rate was 80%. Normal voiding is described as having occurred in all patients. The peak urinary flow rate (PFR, also Qmax) reached a mean of 16 mL/sec immediately after insertion (Poulsen et al, 1993). However, there was a wide range of PFRs, from 4 to 25 mL/sec. Unfortunately, 3 patients later developed urinary retention at 5 days, 2.5 months, and 5 months, respectively, after insertion. Two more stents needed to be removed because of hyperplastic growth of the epithelium. A total of 24 stents were still in place at 3 months. Nordling (1996) updated these authors’ experience after inserting 64 of the modified Memokath stents. Similar successful voiding was reported. The complications that patients described as severe were few, with urgency incontinence being the most common (10 patients). However, moderate symptoms were relatively more common.
Results are available from a long-term study conducted in the United Kingdom. The Memokath was inserted in men who were either permanently or temporarily unfit for TURP, in most cases because of severe respiratory or cardiovascular disease. In this study, 211 men had 217 stents inserted over an 8-year period. In the same time frame, 1511 TURPs were performed. The mean age of patients having stents was 80.2 years, and in the TURP group it was 70.2 years. The patients who had stents fitted experienced an improvement in the mean International Prostate Symptom Score (IPSS) from 20.3 to 8.2 in just 3 months, with results being maintained for 7 years. However, these results must be viewed in the light of the fact that 38% died with stents in place, 34% remain alive with their stents, and 23% had stent removal because of failure. Migration occurred in 13%, and 16% required repositioning. This study suggests that long-term success can be achievable using the Memokath but that failure can also be anticipated in a significant minority (Perry et al, 2002).
The Prosta Coil has been used in a small series of patients with short follow-up (Yachia et al, 1994). The mean follow-up was 14 months, with a range of 2 to 28 months. There were initial irritative urinary symptoms that were reported as having disappeared within 1 month. The mean PFR at the most recent follow-up was 21.3 mL/sec (with a range of 15 to 36 mL/sec) and the mean IPSS was 9 (with a range of 6 to 12).
Polyurethane Stents
Intraurethral Catheter
The intraurethral catheter, the first to be introduced, was reported initially by Nissenkorn (1991). It is made of a type of polyurethane known as Puroflex and has a fixed 16-Fr caliber. Its length varies from 40 to 60 mm, and it can be left in place for up to 6 months. It has a double device at its proximal end shaped like the head of a de Pezzer or Malecot catheter. It has a nylon string at its distal end and a flared split end proximally, which sits in the bladder. It is inserted under topical anesthesia using a 22-Fr cystoscope. The nylon string is cut after placement, and any positional adjustment required can be performed by the use of a grasping forceps. Eighty-five devices were inserted into 73 patients, and, of these, 60 patients had an indwelling catheter for 1 week to 3 years before insertion. Nissenkorn (1991) described a successful outcome in 63 patients who believed that their quality of life was considerably better than it had been when they had an indwelling catheter. He therefore believed that this was suitable for use in such patients, with a high likelihood of success.
A later study reported the use of the Nissenkorn intraurethral catheter in 43 patients (Sassine and Schulman, 1994). Once again, the patients treated had developed urinary retention and were unfit for surgery but also had a short life expectancy. Thirty-six of the 43 patients were able to void satisfactorily after stent insertion. The intraurethral catheter should not be inserted in the presence of bladder stones or anything else likely to block or have a ball-valve effect on the device.
Barnes Stent
What has been called the Barnes stent is made of polyurethane, has a 16-Fr caliber, and is of a single length. It also has a de Pezzer end proximally, but this time a single one. It is thus a modification of the original intraurethral catheter. It was used in 25 patients who underwent endoscopic laser ablation of the prostate (ELAP). Twenty-two of the 25 voided immediately. Early stent migration occurred in 1 patient, but late migration did not occur. The stent was inserted with ease, could be removed with ease at 12 weeks, and was inexpensive. PFRs improved from 8 mL/sec before ELAP to 16.5 mL/sec at 6 weeks with the stent in place (Barnes et al, 1996). The Nissenkorn catheter has also been used safely and with equal success after laser therapy (Nissenkorn et al, 1996).
Trestle Stent
The trestle stent or prostatic bridge catheter has been described and consists of two tubes and an interconnecting thread. The tube that lies in the prostate has a 22-Fr diameter and has a 30-degree angulation. The length is 75 mm, and it has a smooth tip: It is to be used in prostates with a volume of less than 80 mL. The connecting thread is 25 mm, which passes through the distal sphincteric mechanism. The second tube lies in the bulbar urethra and is 35 mm long. It is inserted with the patient under topical anesthesia using a delivery system comprising a positioning stylet, an inflatable balloon with injection cannula, and an outer pusher tube. The technique is described in detail by Djavan and colleagues (1999).
In a report from Devonec and Dahlstrand (1998) the results of its use in 52 patients after high-energy transurethral microwave therapy (TUMT) were described. Tolerance was good in 32 patients, acceptable in 13, and poor in 6. Retrograde ejaculation occurred in eight. PFRs reached 14.6 mL/sec on the day of removal of the device. The device was left in place for 1 month, but the improvement in PFR was maintained at 1 year. Djavan and colleagues (1999) also described its use in 54 patients who had received high-energy TUMT. The device was left in place for up to 1 month, and it was found that the incidence of post-treatment retention was prevented, with concurrent early but significantly improved symptom scores and PFRs. Toleration was high, with 48 of 54 devices remaining in place for 1 month. Early removal was required because of urinary retention in 3 and migration in 3.
The Spanner
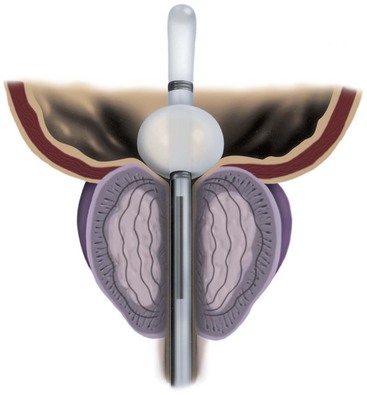
The spanner has a design very similar to the proximal 4 to 6 cm of a Foley catheter. It includes a balloon to prevent displacement, a port for urine drainage that lies proximal to the balloon, and a reinforced stent of varying length that spans most of the prostatic urethra (Fig. 93–1).

Figure 93–1 Pro Vu device used for transurethral needle ablation of the prostate.
(Courtesy of Neo Vitalis Ltd, Southport, UK.)
In the first study using this device, the stent was inserted under topical anesthesia in 30 patients (Corica et al, 2004), of whom 5 had been in urinary retention. The stents remained in place for a mean of 57 days. The mean PFR improved by 42% from 8.2 to 11.6 mL/sec. The overall mean IPSS decreased from 22.3 to 7.1, a 68% difference. The adverse events were few, and the device was found to be stable and patent at the end of the study.
Biodegradable Stents
The concept of stents that can be put in place after a procedure that has a high incidence of secondary and temporary obstruction has been mentioned earlier in the context of stents being inserted after laser or high-energy TUMT. These stents are removed some weeks later. With the biodegradable stent the concept is brought one step further; the stents do not need to be removed, and eventually they disappear by biodegrading. This interesting idea was first introduced in urology when Kemppainen and colleagues (1993) used a biodegradable stent in rabbits after urethrotomy. The idea has now been extended to the ureter (Schlick and Planz, 1998; Lumiaho et al, 1999; Clayman, 2000), after endoscopic urethroplasty (Oosterlinck and Talja, 2000), and in coronary artery disease (Tamai et al, 2000).
Further experimental studies have shown that biodegradable stents can potentially be used as a bridge across the prostate after minimally invasive procedures, without the necessity of having to remove them later (Petas et al, 1997a, 1998; Laaksovirta et al, 2002; Vaajanen et al, 2003).
In addition, clinical studies have been performed that examine the use of biodegradable stents after various procedures (Talja et al, 1995; Dahlstrand et al, 1997; Petas et al, 1997b). The benefit is that these stents prevent the development of obstruction that can occur after laser procedures; however, the use of the second procedure in association with, for example, laser prostatectomy takes away a great deal from the value of the first procedure, not least in terms of cost-effectiveness. There are three types of biodegradable stents.
A randomized study (Petas et al, 1997b) compared self-reinforced polyglycolic acid biodegradable spiral stents (group 1), no device (group 2), or an indwelling catheter (group 3) after visual laser ablation of the prostate. The procedure was performed on 72 men, and 27 were in group 1, 23 in group 2, and 22 in group 3. Voiding began at a median of 1 day in group 1 and a median of 6 days in group 2; the indwelling catheter was required for an average of 6.5 days in group 3, and voiding commenced a median of 6 days after this. The authors found, as they had in previous in-vitro studies, that the stent degraded into small fragments of polymer debris that were passed out in the urine. They commented that voiding became more obstructed at 3 to 4 weeks postoperatively, presumably from degradation and sloughing of the stent, but they found that this was only a transient effect.
In another study, Dahlstrand and colleagues (1997) evaluated the same polyglycolic acid stent after high-energy TUMT with the Prostasoft 2.5. They compared the use of the stent in 15 patients against a further 15 patients in whom a standard 16-Fr urethral catheter was inserted. The mean duration of catheterization was 14.1 days, with a standard deviation of 4.1 days; this was obviously prevented by the stent, which did not cause problems, even when it was degrading.
In a rather innovative way, Knutson and colleagues (2003) described the use of a biodegradable polyglycolic acid stent to assess the risk of post-TURP incontinence in patients with combined BOO and overactive bladder. In 37 patients with severe overactive bladder and moderate to severe BOO this biodegradable stent was inserted into the prostatic urethra; 25 noticed either no leakage or minor leakage and 19 have had TURP with good results. Twelve of the 37 had a major problem with incontinence after the insertion of the stent. There was a small complication rate related to stent insertion.
Permanent Stents
With permanent stenting of the prostate, the urologist is attempting to treat definitively and permanently patients who present with symptomatic BPH. To be of proven value this type of treatment, like any other, must be shown to be at least comparable to TURP. The initial enthusiasm for permanent stents has been replaced by relative silence in the literature at present. Permanent stents were initially introduced as treatment for recurrent urethral strictures and were subsequently used in patients with lower urinary tract symptoms (LUTS). In urologic terms, permanent stents are being used preferentially for the treatment of detrusor-sphincter dyssynergia (Chancellor et al, 1999; Chartier-Kastler et al, 2000; Gajewski et al, 2000), postbrachytherapy BOO (Konety et al, 2000), anastomotic strictures and urinary incontinence after radical prostatectomy (Meulen et al, 1991), and complex urethral strictures (Tillem et al, 1997). There have been no reports in the recent literature that relate to the long-term follow-up of the patients originally treated with permanent stents, and there has been no indication of new interest in their use.
UroLume
Chapple and colleagues (1990) reported on the initial experience with the UroLume. Twelve patients who were considered to be a poor risk for surgery presented with LUTS; 9 of the 12 patients presented with urinary retention. The results were encouraging, with 11 of 12 voiding satisfactorily for a mean follow-up of 8.2 months. The mean PFR after the procedure was 13.6 mL/sec. Further encouragement came from the low complication rate, consisting mainly of short-term irritative voiding symptoms, with only 1 of the 12 being dissatisfied because of severe urgency and frequency (the patient was subsequently found to have detrusor instability). A further study in a similar group of unfit patients was performed by McLoughlin and colleagues (1990). All 19 patients in their study group presented in urinary retention, and all voided satisfactorily after the stent was inserted under local anesthesia.
In a larger, multicenter open trial from the United States, Oesterling and colleagues (1994) reported on 126 men who presented either with moderate or severe LUTS (95 men) or with urinary retention (31 men). There were strict inclusion and exclusion criteria in the trial design, but fitness for surgery was not among them. In the nonretention group, 80 of 95 were evaluable at 12 months and 52 at 24 months; the Madsen symptom score decreased from 14.0 to 5.9 and 5.4, respectively. In the retention group, 24 of the 31 patients evaluable at 12 months had a mean symptom score of 6.1. In the nonretention group, the PFR increased from 9.1 to 13.0 and 13.1 mL/sec, respectively, with the retention group having a mean PFR of 11.7 mL/sec at 12 months. Difficulties with insertion were experienced in 16% of cases; irritative voiding symptoms occurred in 10%.
Guazzoni and coworkers (1994) described a European study using the modified UroLume stent (the so-called less shortening variety described earlier). Once again, the strict inclusion and exclusion criteria did not refer to fitness for surgery, and at this time the stent was being presented as a proposed therapy for prostatic obstruction, not necessarily only for unfit patients. In this multicenter study, 135 healthy patients (91 with LUTS, 44 with urinary retention) were treated. In the nonretention group, 74 of 91 patients were evaluable at 12 months. The mean Madsen-Iversen symptom score had decreased from 14.1 to 6.4, but the tight standard deviations of the mean observed in the U.S. study (0.4) were not seen in this study (5.1); the PFR improved from 9.3 to 15.7 mL/sec at 12 months (with a very wide standard deviation of 6.5, unlike that in the U.S. study). In the retention group, 34 of 44 were evaluable at 12 months; the mean symptom score was 4.5 and the mean PFR was 13.1 mL/sec. The complications were well described but were found to be significant in the long term.
In a British study (Bajoria et al, 1995), 44 men fit for TURP accepted as an alternative a second-generation UroLume stent. The stent was inserted in 44 patients, who either were in urinary retention or had urodynamically proven outflow obstruction. The results achieved were similar to those reported by Guazzoni and colleagues (1994), but there was also a relatively high complication rate. Both sets of authors noted epithelial hyperplasia and migration of the stent in addition to irritative urinary symptoms and painful ejaculation. The second-generation less shortening stent was not recommended for general use, and a third-generation stent was then produced. However, Bajoria and colleagues (1995) strongly suggested that permanent stents should still be considered as being under evaluation rather than for general use.
In a multicenter study of 96 men who were unfit for prostatic surgery, 73 presented in acute urinary retention and 11 in chronic retention. All but 6 were able to void immediately after stent insertion; 2 required a second stent, and 4 required a period of suprapubic catheter drainage. At 12 months, the PFR was 15 mL/sec in the retention group and 18.1 in the nonretention group. Severe irritative symptoms were seen in the majority of patients for up to 3 months, and encrustation was encountered in 15 of 27 patients who underwent cystoscopy (Williams et al, 1993).
The results of a long-term analysis of the UroLume Wallstent have been published by Masood and coworkers (2004). The stent was inserted in 62 patients with moderate or severe LUTS secondary to BPH. The 5- and 12-year follow-up was completed by 22 and 11 patients, respectively. Death occurred in 21 patients (34%), and the stent was removed in 29 patients (47%), the vast majority of these removals occurring in the first 2 years. The authors concluded that this is a safe treatment but that cases must be carefully selected and that it should be performed only by experienced hands.
Memotherm
Williams and White (1995) reported on 48 men with LUTS and urodynamic findings suggestive of bladder outflow obstruction. The results were disappointing. Only 37 patients were able to void immediately after stent insertion, the others requiring a suprapubic catheter for up to 8 weeks. Symptomatic improvement occurred in many, but complications, including stent migration, were relatively high. Thirteen of the 48 patients required removal of their stents. These authors suggested that the results were not appropriate to encourage marketing of the device.
Gesenberg and Sintermann (1998) used the Memotherm in 123 patients considered to be at high risk for prostatic surgery; 46 of these presented in urinary retention. Of the 123 patients, only 52 were evaluable at 12 months. The mean PFR increased from 7.4 to 13.0 mL/sec (with a standard deviation of 6.2), and the IPSS improved from 24.0 to 8.8 (SD 6.2). The authors noted a considerable improvement in quality of life. However, the complication rate was relatively high, with recurrent infections and urgency symptoms in 56%, urothelial hyperplasia in 34%, and urethral stricture in 10%. There was a high number of re-treatments, and the authors suggested that there may be an additional role for medical treatment in some of these patients.
Other Permanent Stents
The ASI stent (Advanced Surgical Instruments) was evaluated in several centers (Kirby et al, 1992; Kaplan et al, 1995). It was introduced on a balloon, which was then inflated, thus expanding the stent. The early results suggested an improvement in symptom score (44%) and PFR (22%), but complications also occurred that made it less attractive for general use. It has since been withdrawn from production.
The Ultraflex stent (Boston Scientific, Natick, MA) is made of nickel-titanium alloy that also has a capacity to expand to a caliber of 42 French when exposed to body heat. It is available in lengths varying from 2 to 6 cm. There have been reports of its use in patients with prostatic obstruction, but it has been studied in a group of patients with detrusor-sphincter dyssynergia (Chartier-Kastler et al, 2000), and the incidence of epithelial hyperplasia and migration was encouragingly low.
Transurethral Needle Ablation of the Prostate
Heat treatment of whatever kind to the prostate is intended to reduce outflow resistance and the volume of the obstruction by increasing the temperature within the prostate and inducing necrosis of prostatic tissue. The aim is to increase prostatic temperature to in excess of 60° C. Transurethral needle ablation of the prostate (TUNA) uses low-level radiofrequency (RF) energy that is delivered by needles into the prostate and that produces localized necrotic lesions in the hyperplastic tissue. It has previously been used to ablate cardiac nerve bundles in the Wolff-Parkinson-White syndrome (Calkins et al, 1992) and to destroy malignant tissue (Rossi et al, 1995; Zlotta et al, 1995). It has also been used to treat chronic cervical zygapophyseal joint pain (Lord et al, 1996). The advantage of TUNA is that it can be delivered under topical anesthesia to patients with symptomatic BPH, causing very precise and reproducible lesions within the prostate.
Delivery of Radiofrequency Energy
RF produces molecular or ionic agitation with collision of particles that relates to the frequency of the energy, and this results in a central hot core inside the prostate and away from the urethra (Schulman et al, 1993). The limited distance dissipation reinforces the safety of the procedure because RF can be applied to tissue only by direct contact, with heat being generated proportional to one over the fourth power of the radius. If the power generated is too high, the prostate rapidly desiccates with a rise in tissue impedance, preventing the desired heating effect. Therefore the appropriate energy level required to produce the localized necrotic lesion must be found, preventing the increase in tissue impedance resulting in prostatic charring around the needle caused by excessive generation of energy (Schulman et al, 1993). Interestingly, heat is lost by convection, and so increased vascularity can have an effect on the degree of localization of the lesion. RF is very much affected by blood flow and has almost no effect on vessels larger than 2 to 3 mm in diameter (Organ, 1976).
There is a difference in the method of tissue heating brought about by RF and, for example, microwave application. Microwaves treat a broad area and can penetrate tissue more deeply than RF. The central temperature is therefore lower than with RF to maintain safe heat levels at the treatment rim. Therefore treatment with microwaves takes longer than RF to produce coagulative necrosis. RF, however, has a much hotter central area with a very quick decline in temperature as the distance increases from the treatment needle. This results in faster generation of the necrotic lesion but of a smaller area (Perlmutter et al, 1993).
Experimental Studies
In a series of preliminary studies on animals and ex-vivo human prostates it has been shown that the TUNA system can create 1-cm necrotic lesions without difficulty in the prostate with no damage to rectum, bladder base, or distal prostatic urethra (Goldwasser et al, 1993; Ramon et al, 1993). Other studies showed that the lesions are accurate with sharp delineation from the untreated areas (Schulman et al, 1993). These authors also showed that the lesion appeared first as a hemorrhagic lesion along the needle path, with slight discoloration in the surrounding area. Necrosis was maximal at 7 days, with fibrosis having developed by 15 days.
In an elegant neurohistochemical study, Zlotta and colleagues (1997) removed prostates from patients scheduled for prostatectomy 1 to 46 days after TUNA. Immunohistochemistry was used with anti–S-100 protein and neuron-specific enolase for nerve staining and anti–prostate-specific antigen and antidesmin for glandular and muscle cells. They showed that the maximal lesion size ranged from 10 × 7 to 20 × 10 mm2 and that there was destruction of all tissue components. The lesions were accurately positioned 0.3 to 1.0 cm from the urethra, which remained undamaged. In the treated area there was an absence of staining for prostate-specific antigen, smooth muscle actin, and α-adrenergic neural tissue. Even in specimens removed 24 hours after TUNA, no positively staining nerve cells were seen in the treated areas.
It has also been shown (Issa et al, 1996) that there may be sequential damage to different types of nerve endings. Nitric oxide synthase receptors were found to be most vulnerable to thermal damage, which occurred earliest, with damage to the α-adrenergic receptors maximal at 1 to 2 weeks.
The temperatures achieved in the largest area have been studied by Rasor and coworkers (1993) using an infrared temperature monitor in an ex-vivo animal model. They showed that the central core of the lesion around the tip of the lesion reached 90° to 100° C. Treatment times of 5 to 7 minutes were required to produce coagulation necrosis in the treatment zone. Dosimetry studies have shown that the temperatures at the edge of the zone were 50° C.
Instruments
The TUNA catheter is, in fact, a specifically designed endoscopic instrument. This has evolved from what was a device through which a panendoscope lens could be inserted. A number of other changes have also been made. The most up-to-date version of the TUNA catheter is called the Pro Vu system, and part of this device is reusable, unlike previous models (Fig. 93–2; see also Fig. 93–1). The new system also contains a markedly improved optical system. Previously, the needles were introduced either blindly or under transrectal ultrasound (TRUS) guidance, which had the advantage in the latter case of seeing how close to the surrounding tissues the needles lay. However, an adequate visualization of the needle entering the prostate tissue gives the urologist a better idea of the treatment area. The final change concerns the angle between the catheter and the needles, which is at present not fixed. This means that a high bladder neck that is hypertrophied or a genuine median lobe enlargement can now be treated easily by this technique.
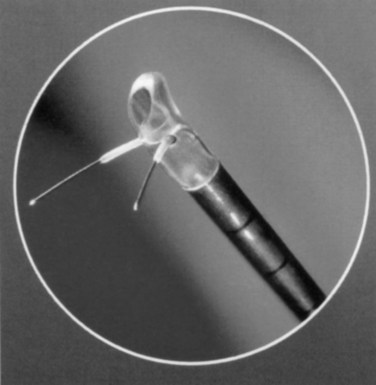
Figure 93–2 Deployment of radiofrequency needles in transurethral needle ablation of the prostate.
(Courtesy of Neo Vitalis Ltd, Southport, UK.)
Clinical Results
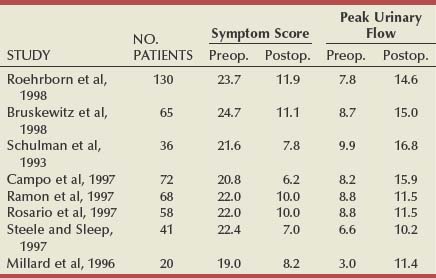
Table 93–1 shows the results of the total world experience in the use of TUNA. There is a wide variety in the number of patients in each series and in the length of follow-up. Of note is that most are open series, with a minority of randomized studies (Issa and Oesterling, 2000). Although the inclusion and exclusion criteria are the same in most series, they are not exactly the same and are not as strict as those in studies testing α-adrenergic blockers and 5α-reductase inhibitors. The size of the studies varies from 12 to 130 patients, and, in many cases, the number of patients observed for the longest period of time consists of less than 50% of the original sample, which makes it difficult to draw definite conclusions. However, when looking at them all together, a number of points can be considered.
A total of 546 patients have been observed for 12 months, with an increase in the mean PFR by an average of 6 mL/sec, representing an average improvement of 77%. The mean symptom score decreased by an average of 13.1 symptom units, which is an average improvement of 58%. Although there was consistency of the general improvements, there was a range. The greatest improvement in the mean PFR was 9.2 mL/sec (Giannakopoulos et al, 1996), and in the mean symptom score it was 15.4 symptom units (Steele and Sleep, 1997). The least improvement in mean PFR was 2.7 mL/sec (Rosario et al, 1997) and in symptom score it was 10.8 (Millard et al, 1996). Although the numbers in patients observed for longer are less (176 for 24 months and 88 for 36 months), the mean improvements appear to be maintained (Issa and Oesterling, 2000).
In the U.S. randomized trial comparing TUNA with TURP, 65 patients were treated by TUNA and 56 by TURP (Bruskewitz et al, 1998). At 1 year, 59 of the TUNA group (90%) and 47 of the TURP group (84%) were available for evaluation. In the TUNA group the symptom score improved from 24.7 to 11.1 (13.6 symptom units) and the PFR from 8.7 to 15.0 mL/sec (6.3 mL/sec). In the TURP group, the symptom score improved from 23.3 to 8.3 (15.0 symptom units) and the PFR improved from 8.4 to 20.8 mL/sec (12.4 mL/sec). The reasons for patients being lost to follow-up are clearly stated, but it was because of ineffectiveness in only 2 patients (3.1%) of the TUNA group and none of the patients treated by TURP. The treatment was found to be effective and safe. The complication rate of TUNA was low, in both the short and the long term; the most commonly reported adverse events were bleeding (32.3%), urinary tract infection (7.7%), and urethral stricture (1.5%). There was no adverse effect of any kind on sexual function in patients treated by TUNA.
There are some additional long-term studies that are of interest. Zlotta and coworkers (2003) entered 188 consecutive patients into a study; there were 5-year data on 121 of these. At the 5-year assessment, 41 of the 176 patients who were evaluable (2 dead, 10 lost to follow-up) required additional treatment after TUNA (23.3%). Although there were 5-year data on 121 patients, and this was defined as a 5-year follow-up, the 10 patients on whom there were only 4-year data were also included, giving a total of 131 patients. The mean IPSS decreased from 20.9 to 8.7, with tight standard deviations but a range at long-term follow-up of 2 to 20. The PFR improved from 8.6 to 12.1 mL/sec with a range at long-term follow-up of 6.5 to 19.2 mL/sec, once again giving the impression of quite a wide scatter of results. Although the drop in the mean IPSS is 12.2, which is an impressive decrease, the improvement in PFR is a less impressive decrease, being 3.5 mL/sec and comparable to that achieved by medical management at the same time point.
In another study from the United States (Hill et al, 2004), 121 men were enrolled in a prospective, randomized, multicenter clinical trial; 65 (54%) were randomly selected to receive TUNA and 56 (46%) were selected to receive TURP. It was reported that 9 of the 65 men (14%) required further intervention in the TUNA cohort, compared with 1 of the 56 men (2%) in the TURP cohort. The requirement for additional medical management in the TUNA cohort was not stated, presumably explaining the difference observed between this study and that described in the previous paragraph. Although this was reported as a 5-year study, only 18 of the 65 men in the TUNA group (28%) and 22 of the 56 (39%) in the TURP were evaluable at 5 years. This makes the 5-year data difficult to interpret despite the highly significant statistical differences described by the authors. At 3 years, the mean IPSS had decreased from 24.0 to 15.2 in the TUNA group and from 24.1 to 10.1 in the TURP group. The mean PFR had improved from 8.8 to 13.0 mL/sec in the TUNA group and from 8.8 to 19.1 mL/sec in the TURP group.
A cost comparison of medical management and TUNA for treating BPH over a 5-year period was performed by Naslund and colleagues (2005). They constructed a cost analysis model using published costs for tamsulosin, finasteride, TUNA, and TURP. They found that over the 5 years tamsulosin was less expensive than TUNA and that finasteride cost the same as TUNA. Combination therapy was more expensive, reaching a break-even point at 2 years and 7 months of treatment. The authors also calculated that TURP is more expensive than TUNA for achieving improvements in IPSS but less expensive for improving PFR.
A meta-analysis of trials of TUNA for treating symptomatic BPH has been reported (Boyle et al, 2004). Meta-analyses are dependent on the data that are put into them. In the case of TUNA, the trials analyzed were often poorly constructed, with inadequate numbers, and not randomized. In fact, there were 2 randomized trials, 2 nonrandomized observational protocols, and 10 single-aim observational studies. One thing that was consistent, however, was that in all studies the patients had severe LUTS, the mean IPSS at entry being greater than 20. The effect of TUNA was to halve the mean IPSS at 1 year. The shortage of long-term studies makes it difficult to draw specific conclusions, but although there was a tendency for the IPSS to increase in the long term the 50% decrease was maintained. The PFR increased by about 70% from baseline to 1 year. It tended to decline over time, but a 50% or greater improvement was maintained. One question has not been answered: although the mechanism of action of TUNA is an adequate explanation for the early improvements, how can it explain any positive long-term effect?
Pressure-Flow Studies
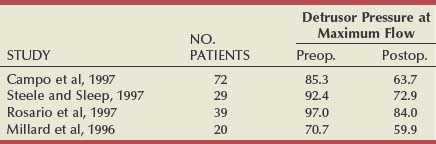
There have been six studies that examined relief of urodynamically proven obstruction as an end point, and these are summarized in Table 93–2. The number of patients in each group varies considerably (from 12 to 108), as does the length of follow-up. The largest number of patients are evaluable at 3 months (253), with smaller numbers having been observed to 12 months (140) or even longer. All patients had pressure-flow studies performed before and after treatment by TUNA.
In another urodynamic study (Minardi et al, 2001), a small number of patients (24) were observed to 24 months. The authors showed that there was initially no change in the prostatic volume or the prostate-specific antigen levels and that pressure-flow studies showed a reduction in the mean opening pressure and Pdet at PFR. This led them to speculate that the ideal patient for TUNA was a man younger than 70 years with a prostatic volume of less than 6 mL, with a pretreatment Pdet at PFR of less than 60 cm H2O and residual volume of less than 100 mL.
Adverse Effects
The adverse effects that occurred in the U.S. randomized study (Bruskewitz et al, 1998) have been alluded to earlier. By far the most common complication reported, however, is post-treatment urinary retention, occurring at a rate between 13.3% and 41.6%. It can be expected that within the first 24 hours about 40% of patients experience urinary retention. The second most common adverse event reported is that of irritative voiding symptoms, occurring in about 40% of patients in the early period after treatment. Given the mechanism of the TUNA treatment, this high rate is surprising, but the symptoms are usually mild, lasting between 1 and 7 days.
Sexual dysfunction is rare after TUNA. Urinary incontinence has not been reported in any series.
Reoperation
The reoperation rate must be compared with that of TURP. Although a 14% requirement for reoperation because of lack of efficacy of the primary treatment with TUNA may seem low, it occurred in less than 2 years (Schulman and Zlotta, 1995). In addition, the 12.7% incidence reported by Steele and Sleep (1997) occurred inside a 2-year period. In the multicenter study reported by Ramon and colleagues (1997), 9 of 76 patients were deemed to have experienced failure of the procedure because of an absence of improvement in PFR. Eight of these patients had no symptomatic improvement, but 5 had an improvement in quality of life.
Indications
The patient most likely to benefit from TUNA would be one who had lateral lobe enlargement and a prostate of 60 g or less (Naslund, 1997). Larger glands can be treated, but more time has to be spent treating each 1-cm segment. Patients with larger prostates, purely bladder neck hypertrophy, or median lobe enlargement are not ideal patients to be treated in this way, but they can be treated; for example, median lobe enlargement can be treated by rotating the TUNA catheter so that the needles point posteriorly, with special care being taken in assessing the depth of their penetration into the prostate.
Transurethral Microwave Therapy
Transurethral microwave therapy has been much evaluated in the past decade and has been widely used. Many urologists have a high regard for its usefulness in treating patients with LUTS, but, for many others, it has no place in the therapeutic line-up. TUMT has been examined clinically in many centers throughout the world, although the very large number of patients who have been entered into open noncomparative studies may surprise some urologists. The rationale for its effect on symptoms has also been studied carefully by authors from different centers, which is unlike that of many of the other so-called minimally invasive treatment modalities. In addition there has been an evolution in the technology of TUMT (Figs. 93-3 and 93-4), from low-energy to high-energy application, perhaps indicating that this technique has a future in the treatment of LUTS.