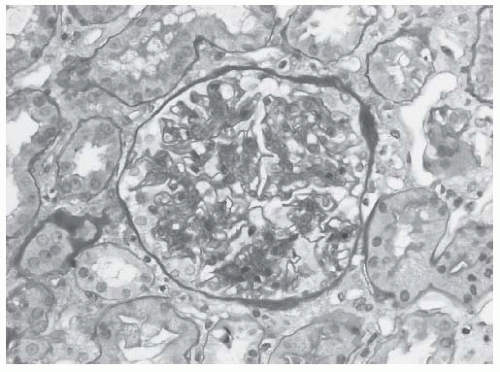
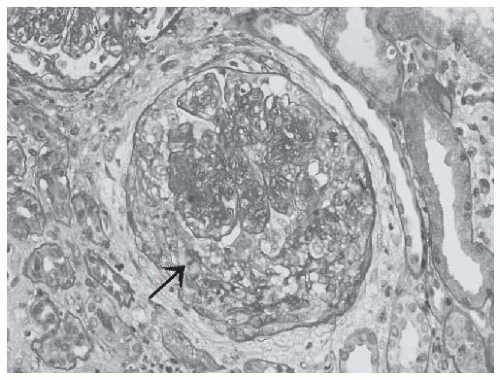
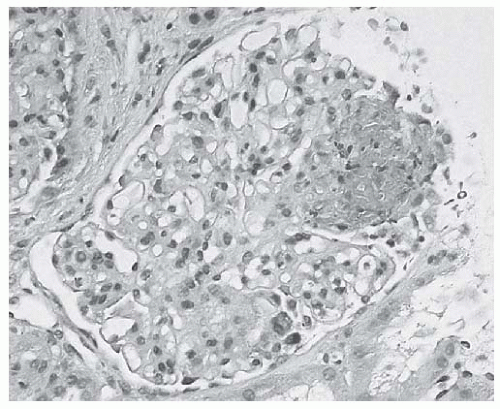
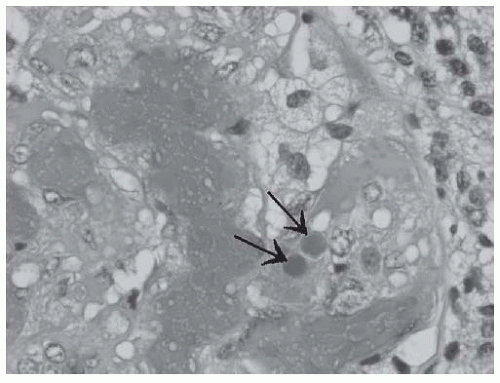
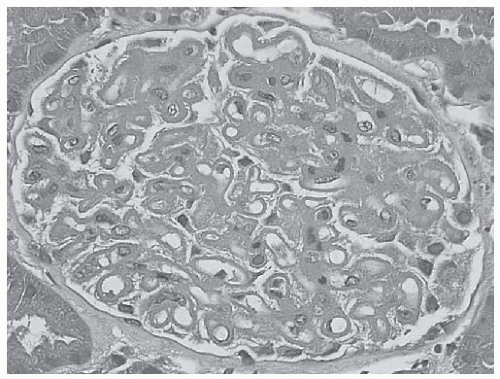
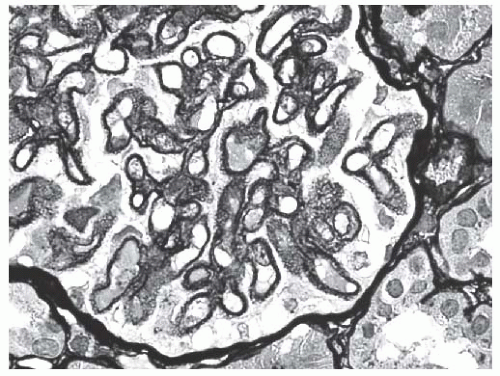
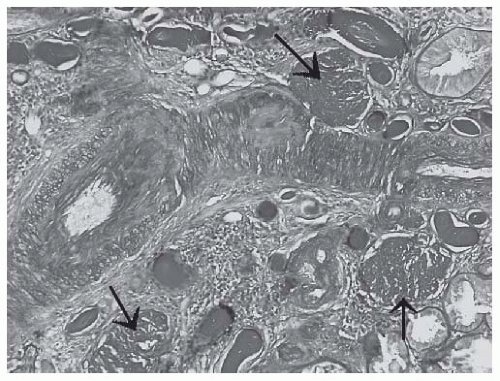
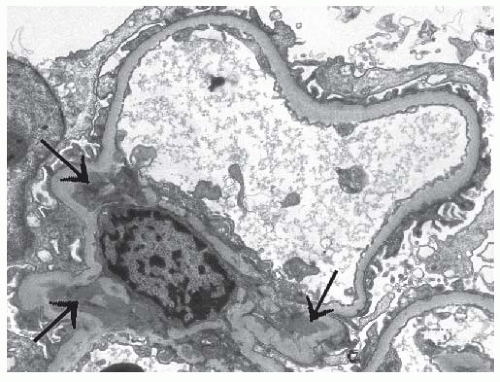
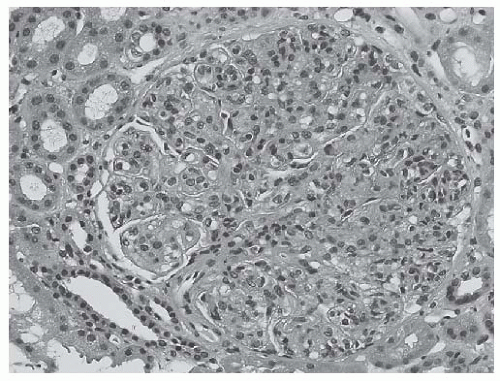
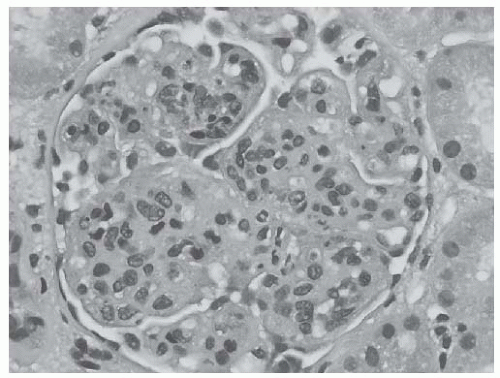
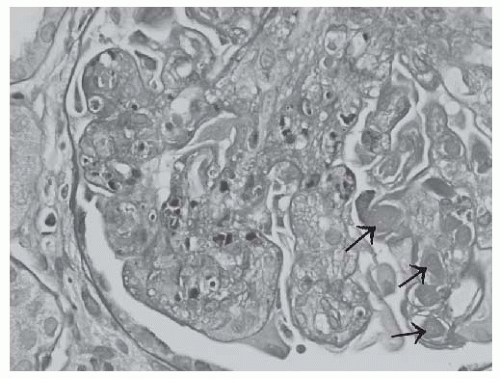
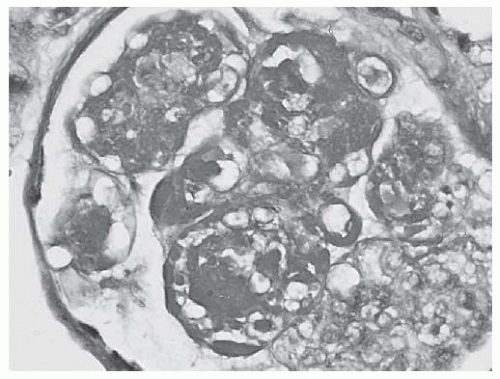
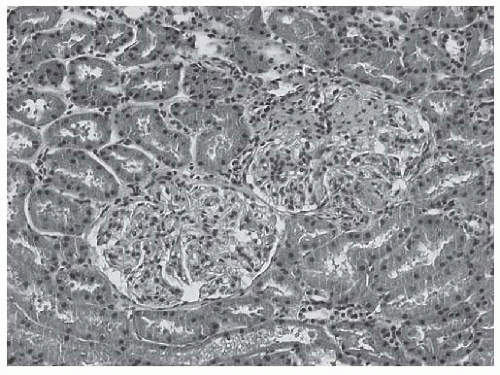
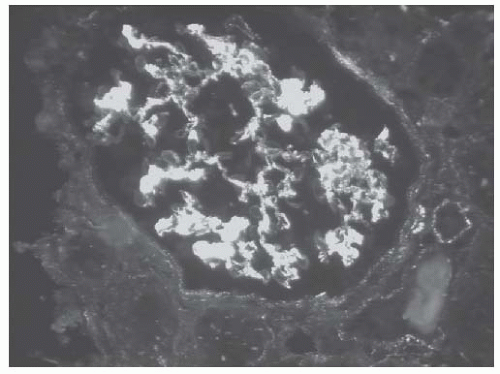
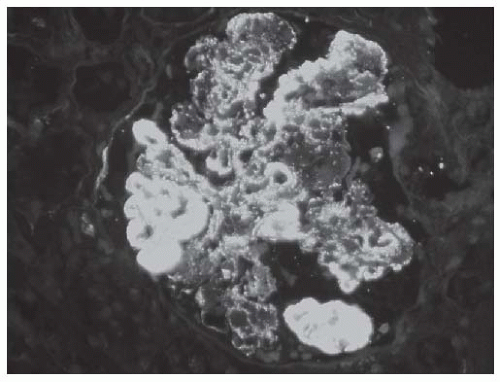
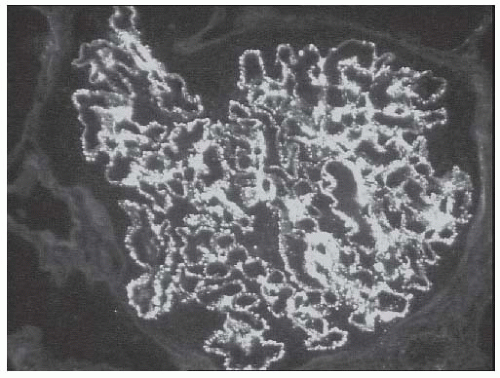
One of the earliest demonstrations of loss of tolerance in SLE was the discovery of autoantibodies in lupus, in particular antinuclear and anti-double-stranded (ds)DNA antibodies.
8 Antinuclear antibodies (ANAs) are the most prevalent, appearing in over 95% of SLE patients. However, over 100 self-antigens have been identified in SLE patients that are targets of autoantibodies, including dsDNA, single-stranded (ss)DNA, nucleoproteins, RNA-protein complexes, ribosomes, phospholipids, carbohydrates, cell cytoplasm and cell surface molecules, blood components, and endothelial cells.
9 The fact that autoantibodies to all of these antigens are not present in every patient suggests that autoantibody specificities may define which organs are affected. Two antibody specificities seem to be particularly relevant to LN
pathogenesis, those against dsDNA and those against the complement component C1q.
Two lines of evidence have historically suggested a specific role for anti-dsDNA in the development of LN. First, numerous studies found an association of high titer anti-dsDNA with active LN.
10 Second, anti-dsDNA antibodies can be isolated from the glomeruli of LN patients.
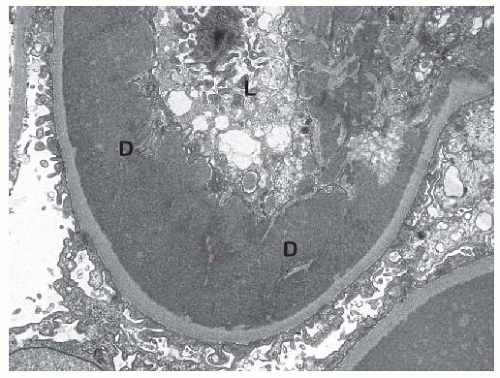
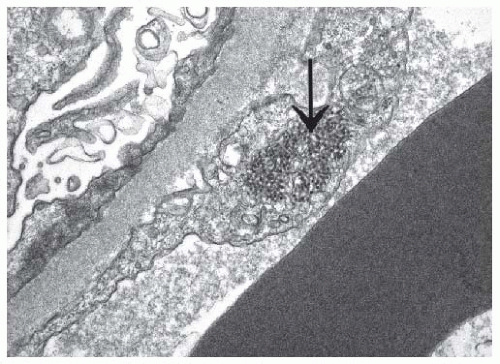
11 Why these antibodies, and IC containing these antibodies, target renal tissue is not completely clear, although two main mechanisms are proposed that focus on the nature of the dsDNA antigen. One mechanism involves nucleosomes, which are composed of DNA in association with a core of positively charged histone proteins. Nucleosomes are released by cells undergoing apoptosis, and can be trapped in the glomeruli, perhaps facilitated by interactions between the positively charged histones and the negatively charged glomerular basement membrane.
12 Anti-dsDNA can recognize the DNA in nucleosomes, and the binding of anti-dsDNA in lupus renal tissue occurs at the site of glomerular nucleosome deposition.
13 Another mechanism is based on cross-reactivity between anti-DNA and one or more renal tissue antigens. Many potential tissue antigens have been implicated, and two of the more relevant candidates are alpha-actinin expressed in both glomerular podocytes and mesangial cells,
14 and annexin II on mesangial cells.
15 Regardless of which mechanism predominates, the result is localized anti-dsDNA-containing IC with the potential to drive local tissue inflammation. Anti-dsDNA autoantibodies appear to be predominantly immunoglobulin G1 (IgG1)
16 which is an inflammatory IgG subtype due to its ability to activate complement and engage Fc receptors for IgG.
Antibodies to C1q, the first component of the classical complement pathway, have been strongly associated with LN in so many studies
17 that some investigators feel they are required for active nephritis.
18 However, this does not seem to be true in all cases.
19 Nevertheless, the high prevalence of anti-C1q antibodies in active LN patients suggests an important pathogenic role. Anti-C1q does not appear to cause an acquired deficiency of circulating C1q because anti-C1q binding requires a neoepitope formed when it becomes fixed to its target substrate. Rather, injury is likely related to interaction of anti-C1q with C1q already present in the kidney, such as in IC bound to nucleosomes.
20,
21 The resulting C1q/anti-C1q IC could focus on inflammatory response to renal tissue, similar to anti-dsDNA/nucleosome IC, leading to nephritis. It should be noted, however, that unlike anti-dsDNA antibodies, most anti-C1q antibodies appear to be IgG2,
22 which is a poor activator of complement and binds Fc receptors with low affinity. Other IgG subtypes (mainly IgG1) can be present in these IC, so the role of anti-C1q in LN pathogenesis may depend on the relative amounts of each anti-C1q IgG subtype.
The Complement and Fcγ Receptor Systems
The formation of IC leads to the activation of both the complement cascade and cells bearing Fc receptors for IgG (known as Fcγ receptors, or FcγR). The activation of complement and FcγR by IC can provide protective effects against SLE, mainly by promoting proper clearance of circulating IC. However, once IC are deposited in tissue, both of these pathways can drive tissue inflammation and damage, either through direct effects on tissue (complement membrane attack complex) or by activating cells to produce proinflammatory cytokines and toxic mediators.
Complement is thought to provide
protection from SLE in a few different ways. First, classical complement activation by IC results in a more soluble, less phlogistic form of IC that is less likely to be trapped in tissue.
23 Second, complement contributes to clearance of apoptotic debris through opsonization by C1q, thus removing a highly immunogenic source of self-antigen.
24 Third, IC opsonization by other complement components (C4b and C3b/bi) that result from complement activation promotes IC clearance through C4b/C3b/C3bi receptors.
25 The type one complement receptor (CR1, CD35), which binds C4b, C3b and C3bi and acts as a regulator of complement activation, is expressed in the circulation predominantly on erythrocytes (E-CR1), and mediates the binding of complement-opsonized IC to erythrocytes (a process known as immune adherence).
26 This binding allows erythrocytes to shuttle IC through the circulation, minimizing glomerular trapping of IC, and promoting IC delivery to the liver and spleen for safe removal.
26 The evidence that all of these complement functions protect against SLE include studies showing that individuals with homozygous deficiencies of classical pathway components have an increased risk for developing SLE and SLE-like diseases,
27 and that E-CR1 levels are decreased in SLE and fluctuate in chronically active disease.
28,
29In contrast, several observations suggest complementmediated inflammation and direct tissue damage contribute to the pathogenesis of LN:
Circulating levels of C3 and C4 are lower in active LN compared to inactive LN or nonrenal SLE, indicating ongoing complement activation.
30,
31
Complement components, including the membrane attack complex, are deposited in LN kidneys.
30,
32,
33
Longitudinal assessment of circulating C3 and C4 levels during SLE flare showed that levels decrease significantly at the time of a renal flare, but not at nonrenal flare, even if the nonrenal flare occurred in patients with a history of LN.
29
Renal tubular production of C3 and complement factor B occurs in LN patients but not healthy controls.
34,
35
The inflammatory receptor for C3a (C3aR), absent from healthy kidneys, becomes expressed in glomerular endothelium in association with IC deposits in LN, and the expression level correlates with LN severity.
36
The inflammatory receptor for C5a (C5aR), although present in normal kidneys, is greatly upregulated in the mesangium and podocytes of LN kidneys.
37
The expression of CR1 is decreased in LN glomeruli, compared to its normal expression on podocytes.
38
The expression of another complement regulator, decay accelerating factor (DAF, CD55), is also reduced in LN patients from its normal expression in the juxtaglomerular apparatus, and appears de novo in the renal vasculature, interstitium, and mesangium.
39
Although there have been no human studies of complement inhibition in LN to verify its pathogenic role, such experiments have been done in experimental animals. For instance, in the NZB/NZW murine lupus model, anti-C5 antibody blocks the development of glomerulonephritis, suggesting C5a and/or the membrane attack complex are critical nephritic factors.
40 In the MRL/lpr mouse model of SLE, the administration of a rodent inhibitor of complement activation (Crry) was effective at protecting against glomerulonephritis.
41 Interestingly, nephritis in the MRL/lpr model appears to be dependent on the alternative pathway of complement activation, as deleting either the factor B or factor D genes significantly reduced the degree of renal injury.
42,
43 The alternative complement pathway is an amplification pathway that is tightly regulated, suggesting that renal damage in LN is due to amplified complement activation occurring in the face of inadequate or overwhelmed complement regulation.
The role of Fc
γR in the pathogenesis of LN, although perhaps not as complex as complement, is similarly confounding. Like complement, IC activation of Fc
γR can provide protection by mediating IC phagocytosis and clearance, but can also induce inflammatory responses by activating the cells expressing Fc
γR.
44 Studies of polymorphic forms of Fc
γR have clarified which role has the most influence in SLE pathogenesis. There are three classes of Fc
γR (Fc
γRI, Fc
γRII, and Fc
γRIII), with different genes that produce full length products for Fc
γRII (Fc
γRIIA, Fc
γRIIB, Fc
γRIIC) and Fc
γRIII (Fc
γRIIIA and Fc
γRIIIB). Single nucleotide polymorphisms (SNPs) that affect the peptide sequence have been identified in some of these genes that influence binding affinity for IgG, including the Fc
γRIIA 491G>A SNP (amino acid 131R>H) and the Fc
γRIIIA 559T>G SNP (amino acid 158F>V).
45,
46 Although not unequivocal, most studies have reported that the lower affinity forms of Fc
γRIIa (R131) and Fc
γRIIIa (F158) are associated with SLE, and particularly with LN.
47,
48 The fact that the forms of these receptors that bind IC more efficiently are associated with protection against SLE suggest that their overall function is to promote IC clearance rather than drive tissue inflammation, and that relative deficiencies in this function contribute to LN.
It should be noted that there is an extensive body of work in mouse models of SLE that suggests IC inflammation is mainly Fc
γR-mediated, with little contribution from the complement system.
49 This includes nephritis in the NZB/NZW model, where deleting the signaling unit of Fc
γRI and Fc
γRIII, which also prevents expression of these Fc
γR, significantly reduces proteinuria, and increases survival time.
50 Although these studies support the potential of Fc
γR to drive inflammation, they do not negate the contributions of complement to this process. Caution must also be taken in their interpretation, as the relative contribution of complement and Fc
γR to mouse models of IC inflammation, including LN, depends on the mouse strain that is being tested.
51,
52 Finally, if the role of Fc
γR, particularly Fc
γRIIIa, in lupus and LN is mainly to drive inflammation, higher affinity forms of the receptor should be associated with worse IC inflammation and LN. However, the studies in human lupus discussed previously demonstrate the opposite; higher affinity forms of Fc
γRIIIa and Fc
γRIIa are associated with protection against SLE and LN. Thus the extent to which these models recapitulate the complex nature of human SLE and LN must be considered.
Renal Chemokines, Cytokines, and Cellular Infiltrates
The presence of IC and the activation of the complement system are key initiators of inflammation that define LN. One consequence of complement activation is the deposition of the membrane attack complex, which directly induces cell membrane damage through the formation of transmembrane pores.
33 Another consequence of IC and complement activation is more indirect, and is mediated by the induction of chemokines and cytokines that induce infiltration and activation of proinflammatory cells. These chemokines and cytokines can be initially produced by renal parenchymal tissue, including glomerular endothelial cells, mesangial cells, podocytes, and tubular epithelium.
53 Once leukocytes containing chemokine receptors are drawn into the kidney, inflammation is accelerated through leukocyte secretion of additional chemokines and inflammatory cytokines. Some notable examples of upregulated chemokines and cytokines in kidneys of LN patients include monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1-alpha (MIP-1
α); interleukin (I)L-6, IL-10, IL-12, IL-17, IL-18; interferon (IFN)-gamma (IFN-
γ); tumor necrosis factor (TNF)-alpha (TNF-
α); and Eta-1/osteopontin.
53,
54,
55,
56,
57In support of a role for chemokines and cytokines in the pathogenesis of LN, deletion or inhibition of their expression substantially reduces kidney injury in mouse models of lupus. For example, deletion of the genes for MCP-1 or its receptor (CCR2) in the MRL/lpr mouse,
58,
59 or predisease treatment of the mouse with a MCP-1 antagonist,
60 reduced infiltration of macrophages and T cells and attenuated clinical and histologic measures of injury, despite accumulation of renal IC comparable to wild-type animals. In both MRL/lpr and NZB/NZW mice, anti-IL-6 antibody treatment reduced anti-dsDNA antibodies and glomerulonephritis, as reflected by near normal renal function and glomerular histology.
61,
62 Anti-IL-18 antibodies, induced in MRL/lpr mice through IL-18 cDNA vaccination, attenuated LN.
63 Anti-TNF-
α treatment of NZB/NZW mice reduced proteinuria, renal inflammatory infiltrates, and glomerulosclerosis, despite increasing circulating anti-dsDNA levels.
64 These data suggest that the renal expression of proinflammatory chemokines and cytokines is an integral step in the pathogenesis of LN.
Some of these may specifically mediate kidney damage (e.g., MCP-1 and TNF-
α), whereas others may predispose to kidney injury through general effects on autoimmunity.
Infiltrating neutrophils and monocytes/macrophages can cause direct renal tissue damage by secreting mediators like reactive oxygen species and proteolytic enzymes. The effect of infiltrating T cells is less direct, and is reflected by the cytokine profile of these T cells. During proliferative LN the intrarenal production of Th1 cytokines appears to predominate over Th2 cytokines and correlates with histologic activity. Th1 responses are associated with activated macrophages, and with the production of IgG capable of activating complement and Fc
γR pathways. Specifically, relatively high levels of IL-12, IFN-
γ, and IL-18 are present, although IL-10, a Th2 cytokine, has also been shown to increase. This leads to an overall higher Th1/Th2 cytokine ratio.
55,
56,
65,
66 Th1-dominant expression can also be observed in serum, urine, and circulating T cells of LN patients.
66 The Th1 dominance displayed in LN patients, both locally in the kidney and systemically in the circulation, suggests that this may be an important prerequisite for developing LN.
IL-17 may also play a particularly important role in the pathogenesis of LN. As mentioned previously, IL-17 is found in the kidney in LN, and two major cell sources of IL-17, Th17 cells and CD4-CD8 T cells, have been observed in renal biopsies of LN patients.
57 Local production of IL-17 may drive inflammatory cytokine and chemokine expression by resident glomerular and tubular cells having the IL-17 receptor,
67 leading to activation of neutrophils and monocytes.
68 The presence of IL-17-producing cells in the LN kidney may also represent a shift away from natural regulatory T cells capable of suppressing immune responses.
69 The role of regulatory T cells is discussed later.
Although not usually prevalent, infiltrating B cells have also been described in LN kidneys. Their presence may directly target autoantibodies to the kidney, as has been shown in NZB/NZW mice.
70 B cells in renal tissue may also present kidney antigens to intrarenal T cells. Recent work has shown that intrarenal B and T cells associate with various degrees of organization, including structures resembling germinal centers with central follicular dendritic cells.
71 Interestingly, these structures appear to occur mainly outside of the glomeruli, and are associated with tubular basement membrane IC.
71 These may contribute specifically to tubulointerstitial inflammation in LN.
Intrinsic Regulatory T Cells
Human regulatory T cells (Treg), characterized as CD4
+ CD25
hiFoxP3
+, inhibit immune responses through effects on T and B cells, and particularly autoantibody production.
72,
73 Studies in the NZB/NZW mouse suggest a role for Tregs in lupus pathogenesis, with an inverse correlation between circulating Treg numbers and circulating anti-dsDNA levels,
74 and suppression of lupus-like disease activity, including glomerulonephritis by adoptive transfer of Tregs.
75 More than 25 studies have been done on human SLE and the majority of these indicate lower circulating levels of Tregs in SLE, although there is no clear consensus.
76 With regard to the role of Tregs in human LN, one study demonstrated an increase in Treg markers following rituximab-induced B cell depletion in LN patients (
n = 7) that correlated with clinical remission,
77 whereas a second study showed no relationship between circulating Treg numbers or function and active LN.
78 Although Tregs are likely involved in SLE pathogenesis, the specific nature of that involvement, especially with respect to LN, remains to be determined.
Interferon-α and Plasmacytoid Dendritic Cells
IFN-
α has recently taken a central role in the proposed paradigms of SLE pathogenesis.
79 This pathway is initiated when IFN-
α is produced in response to a variety of stimuli, most involving nucleic acids. Plasmacytoid dendritic cells (pDCs) are the major sources of IFN-
α following engagement of their endosomal toll-like receptors 7 and 9 (TLR7, TLR9) by ssRNA and unmethylated CpG in DNA, respectively.
80,
81 Both TLRs are intracellular. Other cell types can produce IFN-
α following engagement of different receptors, such as TLR3 in myeloid dendritic cells, or non-TLR pattern recognition receptors such as the helicases RIG-I and MDA5 in a variety of cells.
82 All these receptors sense various viral and bacterial nucleic acids and activate signaling cascades that end in the production of IFN-
α. The effects of IFN-
α on the immune response includes driving maturation of conventional dendritic cells into potent antigen presenting cells,
83 inducing B cell differentiation to plasma cells,
84 and contributing to the development of CD4 helper T cells
85 and CD8 central memory T cells.
86The IFN-
α response receptors theoretically are important in discriminating between self and nonself. For example, TLR7 shows specificity for guanosine/uridine rich ssRNA such as viral ssRNA, whereas TLR9 shows specificity for unmethylated CpG that occurs mainly in nonmammalian DNA. Both receptors also can recognize mammalian nucleic acid in the form of IC containing RNA/protein (e.g., anti-RNP IC) or anti-dsDNA containing IC.
87 The presence of autoantibody may be crucial for this recognition, as RNA and DNA in the form of IC allow phagocytosis of the nucleic acids via Fc
γRIIa expressed on pDCs.
88 By generating increased IFN-
α through this mechanism, an enhanced immune response can occur that may break tolerance to RNA and DNA-containing antigens, resulting in the types of autoantibody that are prevalent in SLE. Initiation of SLE strictly by this mechanism would require a baseline level of IgG against nucleic acids, which is reasonable as ANA positivity occurs in >1% of the general population.
89 Whether the IFN-
α /pDC pathway initiates SLE or not, evidence suggests that the pathway is important to the pathogenesis of SLE. This evidence includes the observation that patients treated with IFN-
α can develop a lupuslike disease,
90,
91 the identification of a number of IFN-
α-related
genes as susceptibility genes for SLE onset,
79 an increase in IFN-
α induced gene expression (the IFN-
α signature) associated with active SLE,
92 and the number of known SLE autoantigens that can drive IFN-
α secretion.
There is also evidence that IFN-
α may be particularly involved in the pathogenesis of LN. Serum levels of IFN-
α correlate directly with anti-dsDNA and inversely with C3 levels,
93,
94 markers that are associated with LN. Peripheral blood cell levels of the IFN-
α signature are associated with LN patients.
92,
95 IFN-
α-inducible chemokines, including MCP-1, correlate negatively with C3 levels, and are associated with active LN,
94 and with risk for renal flare.
96 During severe LN pDC disappear from the circulation and accumulate in glomeruli, due in part to glomerular expression of IL-18 and pDC expression of the IL-18 receptor.
97 It is plausible that the presence of renal IC containing dsDNA (e.g., nucleosomes) could drive glomerular pDCs to produce IFN-
α, thus amplifying the autoimmune response to local glomerular antigens and contributing to the formation of local germinal centers. Studies in mouse models also generally support a role for IFN-
α in LN pathogenesis. Experimental LN is reduced by deletion of the IFN-
α receptor or by administration of TLR7 or TLR9 antagonists, whereas LN is worsened by administration of an IFN-
α-producing vector or an agonist of TLR7 or 9.
98 One exception is seen in the MRL/lpr model, in which LN is significantly worsened following deletion of the IFN-
α receptor,
99 suggesting that IFN-
α protects against LN in this mouse strain.
The realization of the importance of the IFN-α pathway in SLE pathogenesis has reinvigorated the concept of microbial pathogen involvement in SLE pathogenesis. The activation of TLRs and other sensors that stimulate IFN-α by viral and bacterial nucleic acids may be important in initiating the break in tolerance, or in accelerating the autoimmune response.
The Genetics of Lupus Nephritis
Much effort has gone into identifying the basis for genetic susceptibility to SLE, using genomewide and candidate gene studies.
100 Over 30 genes have been identified that appear to be related to specific pathogenic pathways in SLE. These include IC clearance/inflammatory pathway genes, immune response genes, and IFN-
α signaling and response genes. A number of these impart particular susceptibility to LN.
101 Examples include genetic variation in the Fc
γRIIA and Fc
γRIIIA genes described previously, in which the higher affinity variants are associated with protection against LN.
47,
48 Two cytokines previously discussed as important for cell infiltration into the kidney, the chemokine MCP-1 for monocytes/T cells and IL-18 for pDCs, have promoter polymorphisms that influence expression levels. The MCP-1 variant that results in higher expression levels is associated with LN.
102 Similarly, the IL-18 variant that causes higher expression is associated with diffuse proliferative LN.
103 Also of interest, the HLA DR3 allele (DRB1*0301) correlates with renal disease,
104 and with anti-dsDNA antibodies,
104 supporting a genetic contribution to a type of autoantibody that may target renal tissue. For the IFN-
α pathway, STAT4, which is important for transmitting the IFN-
α signal, has a genetic variant that is associated with increased STAT4 RNA levels, and with SLE, particularly LN.
105Genome studies have identified six quantitative trait loci (QTLs) that are linked to LN, supporting the fact that LN has a specific genetic component.
106,
107 Three of these regions are linked to LN in European Americans, and three are linked to LN in African Americans. One of the loci for European Caucasians occurs on chromosome 4, at q13.1, a region that contains the gene for IL-18. This may account for the relationship between this QTL and LN.
A Composite Picture of Lupus Nephritis Pathogenesis
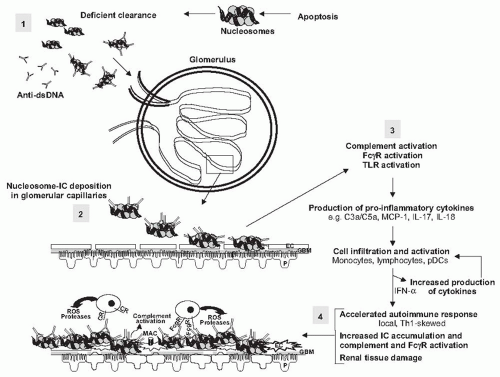
Considering all of the LN-specific “traits” of the various pathways that contribute to SLE pathogenesis, a picture emerges as to what may be the important steps that culminate in clinical LN (
Fig. 53.1). Clinically active LN is always associated with IC accumulation and complement deposition in the kidneys, and often with corresponding evidence of systemic complement activation. The IC that are perhaps most relevant to LN are those containing nuclear antigens. These can arise due to deficiencies in clearance of IC containing nuclear antigens from microbes or apoptotic debris. Deficiencies in the clearance of apoptotic debris may also lead to glomerular accumulation of self-antigen, such as nucleosomes, that can target autoantibody directly to renal tissue. Initial accumulation of glomerular IC sets the stage for an escalating cascade of events that includes local complement activation and chemokine/cytokine production, leading to infiltration and activation of inflammatory (monocytes, neutrophils) and immune cells (pDCs, T cells), and a heightened intrarenal Th1-dominant immune response with significant Th17 contributions. This then leads to an escalation of autoantibody production targeted to the kidney, and inflammation driven primarily by complement and Fc
γR activation. Many of the mediators derived from this activation contribute to kidney injury, including direct tissue damage by complement proteins and toxic factors produced by inflammatory sells, such as reactive oxygen species and proteolytic enzymes. Continued inflammation can lead to matrix expansion, fibrosis, scarring, and eventually ESRD.
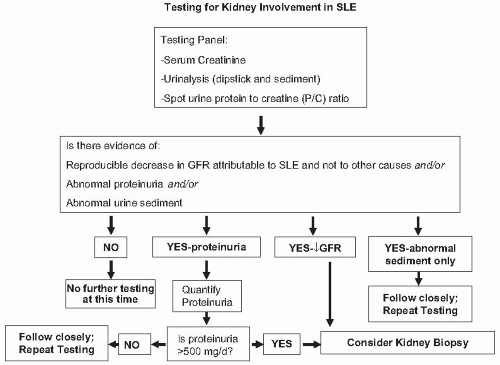
Why LN occurs only in some SLE patients remains an unknown, although the data discussed previously point to the existence of specific LN genes, including those that favor inefficient IC clearance, exuberant chemokine/cytokine production, and loss of tolerance and activation of T and B cells. Environmental contributions such as exposure to certain microbial infections may also be involved in the development of LN. As the specifics of how genetic
and environmental factors interact and contribute to LN become clearer, so too will our understanding of the pathogenesis of LN.