Alexander C. Wiseman, James E. Cooper
Prophylaxis and Treatment of Kidney Transplant Rejection
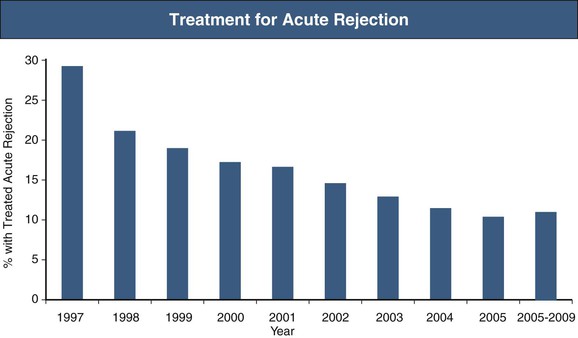
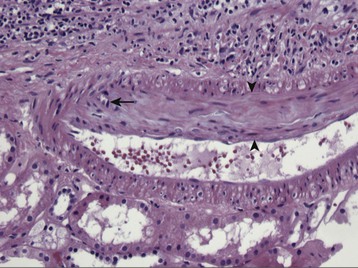
The clinical presentation of the immune response to transplanted tissue, referred to as rejection, became apparent in 1960 when, after successful proof-of-principle kidney transplants were performed in identical twins, kidney transplantation was attempted between immunologically dissimilar individuals.1 Eleven patients underwent lymphoid irradiation to prevent rejection after kidney transplant from nonidentical donors. Although 10 of the 11 died of overwhelming infection, illustrating the potential consequences of immunosuppression, the lone surviving patient from this series subsequently underwent two episodes of acute rejection, both of which were successfully treated with corticosteroids with successful graft function. Thus began the development of immunosuppressive agents that could prevent and treat rejection while not inducing severe life-threatening side effects, and the characterization of the histologic patterns of injury that quantify the type and severity of rejection. The occurrence of acute rejection in the first year after transplant has significantly diminished from a near-universal occurrence of rejection in earlier eras to present-day rates of 10% to 15%,2 primarily because of the development of newer immunosuppression medications. Although the incidence of acute rejection has diminished, the management of chronic rejection has remained a challenge, with continued attempts to better define the nature of injury and methods to prevent or reverse this process (Fig. 104-1). However, as attention shifts to limiting the toxicity of immunosuppression medications in corticosteroid-withdrawal and calcineurin inhibitor (CNI)–withdrawal protocols and to attempting to increase access to transplant by performing transplants across human leukocyte antigen (HLA) and blood type barriers, management of acute rejection continues to be an important clinical issue.
Definition
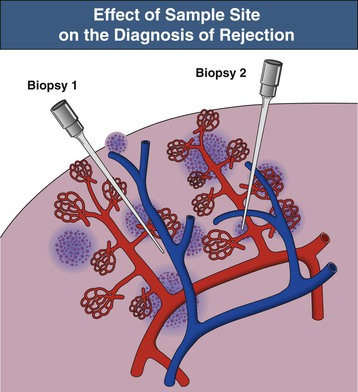
Rejection (both acute and chronic) is defined by histologic findings after kidney transplant biopsy. A biopsy considered adequate for analysis involves sampling of at least 10 glomeruli and two small arteries, stained with hematoxylin-eosin (HE), periodic acid–Schiff (PAS) or silver, and trichrome stains; a biopsy with seven to nine glomeruli and one artery is considered of marginal adequacy. When biopsy is performed for clinical indications (renal dysfunction), two separates cores should be obtained because the findings of rejection are often patchy in distribution (Fig. 104-2).3 There is not a consensus of opinion regarding the number of cores required when biopsies are performed for nonclinical indications (e.g., in protocol-driven practice), although adequate tissue sampling defined by the previously described criteria is preferable if performed.
The Banff Working Classification of Renal Allograft Pathology forms the basis of the histologic definition of rejection and is continually reviewed and updated on a biannual basis. First developed in 1993 with a primary focus on T cell–mediated acute inflammatory infiltrates to classify the degree of rejection, a more recent (2007) classification now differentiates a humoral (antibody-mediated) response from the T cell response and further distinguishes a chronic humoral form of injury previously included within the term “chronic allograft nephropathy” (see Chapter 107)4 (Box 104-1). This was based on the indirect identification of antibody-mediated injury via evidence of complement (C4d) deposition. C4d is a fragment of C4b that is generated on IgG and IgM deposition and activation of the classical complement pathway. C4b/C4d forms a covalent bond with proteins on tissue such as capillary endothelial cells via a sulfhydryl group, and persists bound to tissue after immunoglobulin and other complement products have been released.5 Staining for C4d, either by immunohistochemistry or immunofluorescence, is often performed on allograft biopsy specimens to aid in identifying if an antibody-mediated process may be involved in patients with graft dysfunction. Although issues of standardization and reliability of staining exist, C4d staining remains an important marker of antibody-mediated injury and should be performed in any biopsy from which a change in immunosuppression may be entertained.
Antibody-Mediated Rejection
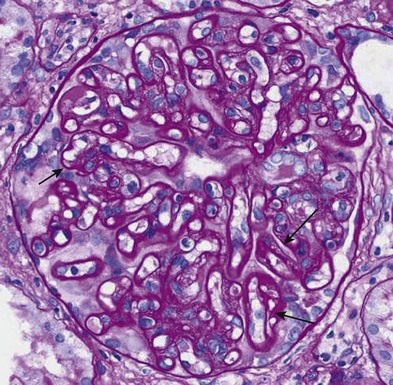
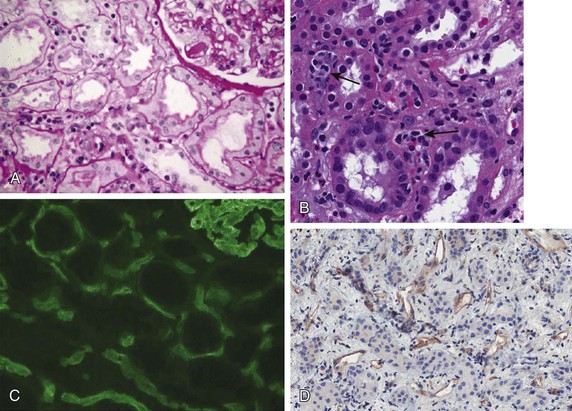
Acute antibody-mediated (humoral) rejection (AHR) is estimated to occur in 3% to 10% of all transplants and is present in 20% to 30% of episodes of acute rejection,6 occurring typically within the first few weeks of transplantation or in association with a change in immunosuppression. Although patients who have pre-existing donor-specific HLA alloantibodies (“donor-specific antibodies”) are at higher risk for the development of acute humoral rejection, the identification of de novo donor-specific antibodies at the time of graft dysfunction is common. The diagnosis of acute humoral rejection requires (a) evidence of circulating donor-specific antibodies, (b) C4d deposition in peritubular capillaries, and (c) evidence of tissue injury (Fig. 104-3). Patterns of injury associated with acute humoral rejection range from acute tubular cell injury suggestive of acute tubular necrosis to thrombotic microangiopathy (TMA), but typically will be associated with neutrophils and/or macrophages in peritubular capillaries. Staining for C4d is considered a sensitive marker for acute antibody-mediated injury and should be routinely performed on renal allograft biopsy specimens when this process is being considered in the differential diagnosis.
Chronic active antibody-mediated rejection has been identified as a leading cause of late allograft loss,7,8 and accurately diagnosing this condition has been a subject of interest. Chronic active antibody-mediated rejection is likely the result of an indolent alloimmune response that can result in transplant glomerulopathy and microcirculatory inflammation (Fig. 104-4). Although transplant glomerulopathy is often associated with circulating donor-specific antibodies and C4d deposition, 30% to 50% of cases will be identified in the absence of these diagnostic markers.9 This suggests that either these lesions are not solely caused by a humoral response or the lack of a temporal relationship of donor-specific antibodies or C4d deposition to biopsy findings is related to phenomena such as non–complement fixing antibodies and/or the waxing-waning nature of the humoral response.
The current Banff diagnostic criteria for chronic humoral rejection require (1) evidence of donor-specific antibodies, (2) C4d deposition in peritubular capillaries, and (3) evidence of chronic tissue injury. The forms of chronic tissue injury may include duplication of the glomerular basement membrane, multilamination of the peritubular capillary basement membrane, arterial intimal fibrosis without elastosis, and/or interstitial fibrosis with tubular atrophy. Although the Banff classification still recognizes diffuse peritubular capillary C4d deposition as a diagnostic criterion for chronic active antibody-mediated rejection, recent evidence questions the sensitivity of C4d in detecting humoral graft damage and suggests that microcirculatory injury (peritubular capillaritis, glomerulitis) may be a more important indicator of this process.7,10 Recent molecular studies show upregulated endothelial gene expression in combination with circulating donor-specific antibodies to be indicative of antibody-mediated graft damage (termed C4d-negative antibody-mediated rejection) and a more sensitive indicator of graft loss than C4d deposition.11 A report from the 2011 Banff meeting recognizes the existence of C4d-negative antibody-mediated rejection and has aimed to define diagnostic criteria for this entity.12
T Cell–Mediated Rejection
The classical pathologic description of rejection is now referred to as T cell–mediated rejection. The classification of acute T cell–mediated rejection (acute cellular rejection [ACR]) is based on the degree and location of mononuclear cell inflammation. Because interstitial inflammation and tubulitis are frequently present immediately beneath the renal capsule (subcapsular inflammation) in stable allografts, this histology is not taken into account in interpreting an allograft biopsy for the presence of rejection. When severe, interstitial inflammation may extend into tubules via injury to the tubular basement membrane (tubulitis). The predominant phenotype of these infiltrates is a mixture of CD4+ and CD8+ T cells; however, B cells, eosinophils, and macrophages may also be present. Less commonly, endarteritis (endothelialitis) may be present: T cells and macrophages extend under the arterial endothelium, a phenomenon that may or may not be accompanied by interstitial inflammation or tubulitis. The finding of interstitial infiltrates and tubulitis in a kidney transplant biopsy specimen is not specific to ACR, and other causes such as viral nephropathy (BK virus, less commonly cytomegalovirus [CMV]), pyelonephritis, or post-transplant lymphoproliferative disease should be considered based on the clinical presentation. In contrast, the histologic finding of endothelialitis is pathognomonic of ACR.
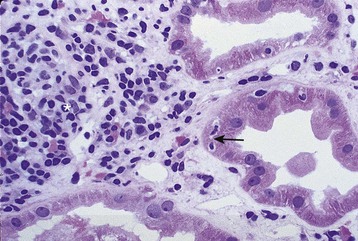
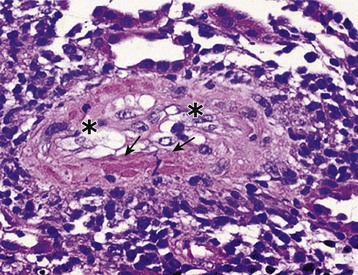
Acute T cell–mediated rejection is histologically classified in the Banff criteria based on the presence or absence of endothelialitis, the degree of interstitial inflammation, and the quantity of infiltrating cells into tubules. Type I ACR is characterized by the absence of endothelialitis, with interstitial inflammation of at least 25% of the parenchyma, and tubulitis (type IA requires 4 to 10 mononuclear cells/tubular cross section, whereas type IB requires >10 mononuclear cells/tubular cross section) (Fig. 104-5). Type II ACR is characterized by vascular involvement or endothelialitis (IIA requires mild to moderate intimal arteritis, and IIB requires arteritis with at least 25% of the luminal area lost in at least one arterial cross section) (Fig. 104-6). Type III ACR is characterized by vascular inflammation that extends to the media (“transmural”) and may be accompanied by fibrinoid change and necrosis of the smooth muscle cells (Fig. 104-7). Type II and III ACR may or may not be associated with elements of type I ACR; therefore the pathologic description of rejection should not be viewed as a pathogenic continuum. However, type II and III ACR appear to require different therapeutic interventions and carry different prognostic implications than type I ACR (see later sections).
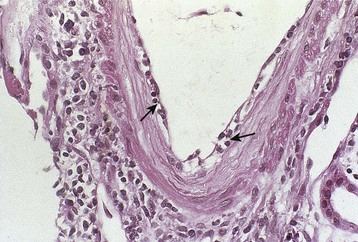
Chronic active T cell–mediated rejection is a histologic diagnosis that refers to arterial intimal fibrosis specifically with evidence of mononuclear cell infiltration and formation of neointima (Fig. 104-8). This is distinguished from chronic humoral rejection by the location of vascular injury and lack of evidence of pathogenic antibody, and is distinguished from other nonimmunologic processes that may lead to vascular and interstitial fibrosis by the presence of persistent infiltrating cells within vessels. This is covered in greater detail in Chapter 107.
Borderline Rejection
The finding of inflammation in 10% to 25% of the interstitium with tubulitis of less than four mononuclear cells per tubular cross section is classified as borderline rejection. This currently remains a pathologic definition without clear clinical significance. When borderline rejection is identified in the setting of graft dysfunction or with other findings such as glomerulitis, the risk of progression to clinical rejection on subsequent biopsies is increased and therefore treatment may be considered.13
Clinical Manifestations
The clinical presentation of acute rejection is common to both T cell and antibody-mediated rejection. Patients typically present with a rapid rise in serum creatinine, and in severe cases may have a decreasing urine output, weight gain, fever, or graft tenderness. The clinical findings are commonly nonspecific, and other causes of graft dysfunction are often considered at the time of presentation in the context of an individual’s risk for the development of acute rejection (Boxes 104-2 and 104-3). Because of the increase in presensitized patients undergoing transplantation, patients with known donor-specific antibody in desensitization protocols, and ABO-incompatible transplants, approximately 25% of acute rejection episodes now have a humoral component. In acute humoral rejection there may be features of TMA with microangiopathic anemia and thrombocytopenia. If there is immediate cyanosis of the graft on revascularization (hyperacute rejection) or an abrupt decline in urine output and graft tenderness 3 to 14 days post-transplant (delayed hyperacute or accelerated rejection), donor-specific antibody is implicated. Typically there is type III ACR and interstitial hemorrhage on biopsy.
Prophylaxis and Prevention
Prophylaxis
The primary goal of transplant management is prevention of immunologic graft loss in the early period after transplant. Over time, the risk for acute rejection diminishes and goals of immunosuppression therapy shift toward considerations of side effects of medications and risks for other events such as cardiovascular disease and malignancy. Therefore, current clinical practice follows a general strategy of intensive immunosuppression and monitoring in the first months after transplant with a reduction or alteration of treatment after the initial period of risk.
Prevention of Acute T Cell–Mediated Rejection: Induction Therapy
The use of a brief course of potent immunosuppression at the time of transplant, referred to as induction therapy, has become a common strategy for the prevention of acute rejection in all transplant recipients including both those at higher and lower immunologic risk. According to the Scientific Registry of Transplant Recipients (SRTR), 83% of kidney transplant recipients received induction therapy in the United States in 2011. For higher-risk patients such as those with prior sensitization (the presence of HLA antibodies quantified by percent reactivity against a panel of common HLA types, referred to as percent panel reactive antibody [PRA]), prior transplant, or African American ethnicity, induction therapy is usually combined with standard doses of immunosuppression to prevent rejection. For those with lower risk (living donor kidney recipients, primary kidney transplants), induction therapy is often used in an effort to minimize exposure to maintenance immunosuppression. The use of race as a risk factor for rejection has recently been questioned with a study demonstrating similar acute rejection rates in African Europeans compared with European Caucasians in France.14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree