Although surgical treatment is curative for localized renal cell carcinoma (RCC), 25% of patients present with locally advanced or disseminated disease, and disease will recur systemically in another 20% to 30% of those who have localized disease at presentation. Many clinical, histologic, and molecular factors have been identified that place patients who have localized RCC at greater risk for recurrence and those who have metastatic disease at risk for progression or death. This article reviews the major prognostic factors for RCC and the most commonly used algorithms developed for use before or after nephrectomy and before initiation of systemic therapy. These RCC nomograms allow more accurate counseling of patients regarding their likely clinical course and facilitate treatment planning.
Renal cell carcinoma (RCC) responds minimally to conventional chemotherapy and remains the most lethal of the common genitourinary cancers. Cancer-specific survival correlates strongly with tumor stage, although several other factors have been shown to be independent predictive factors of disease recurrence. Long-term survival exceeds 90% in patients who have RCC that is contained within the kidney (pathologic stage T1) but is less than 5% in patients who have metastatic RCC. Although surgical treatment is curative for localized disease, 25% of patients present with locally advanced or disseminated disease, and disease recurs systemically in 20% to 30% of patients who have localized disease at presentation. Only a small percentage of patients respond to interleukin-2– or interferon-based immunotherapy, and these treatments can be associated with considerable toxicity. Unfortunately, the incidence of all stages of RCC continues to increase by about 2.5% per year, and RCC was responsible for more than 12,800 cancer-related deaths in the United States in 2007. The pressing need for effective systemic therapies has led to the development of several novel treatments for metastatic RCC that are now available for clinical use. Two oral, small molecular inhibitors of the receptor for vascular endothelial growth factor (VEGF) have been approved recently by the Food and Drug Administration for the treatment of advanced RCC. Sunitinib (Sutent) and sorafenib (Nexavar) exert their anti-cancer effects primarily by blocking pathways induced by the binding of VEGF to VEGF receptor, leading to the growth of new blood vessels (angiogenesis) that supply oxygen and nutrients to the growing cancer. These agents have been shown to result in stabilization or a decrease in tumor burden in a majority of patients. They also have been shown to lengthen the time to disease progression when compared with placebo or interferon treatment. Temsirolimus, an inhibitor of the mammalian target of rapamycin, is a third agent recently approved by the Food and Drug Administration that has been evaluated in poor-risk patients who have metastatic RCC. When administered intravenously to this patient subgroup, temsirolimus extended recurrence-free and overall survival when compared with interferon in a randomized, controlled trial.
Each of the currently available treatments, as well as those currently in clinical trials, is costly and is associated with side effects. Therefore, it is important to identify patients who have the greatest likelihood of benefiting from such treatments. Determination of the risk of recurrence in patients without evidence of metastasis has been based on several predictive models that integrate known clinical risk factors for disease recurrence. More recent studies suggest that the addition of molecular markers to clinical risk factors may improve the predictive ability of RCC disease-recurrence models. Prediction of disease progression or death from disease also has been investigated for use in counseling patients who have metastatic RCC and for guiding clinical decision making. This article describes the various predictive factors and algorithms for patients who have localized and metastatic RCC.
TNM staging system
Until 1990, Robson’s modification of the staging system proposed by Flocks and Kadesky provided the most reliable prognostic information for clinicians caring for patients who had RCC. The system most commonly used currently is the TNM staging system of the International Union Against Cancer and the American Joint Committee on Cancer. Tumor stage remains the single best prognostic indicator for RCC, although it may be viewed better as an algorithm that combines several factors, each of which provides information about outcome. Two studies have confirmed that the 2002 modification of this system has better prognostic ability than the previous 1997 staging system, and a new staging system will become available soon. Modifications in the 2002 staging system included subclassification of pT1 tumors as pT1a (< 4 cm) and pT1b (4–7 cm), grouping extension into the renal vein and inferior vena cava below the diaphragm as T3b, and re-classifying involvement of the inferior vena cava above the diaphragm or invasion of the inferior vena cava wall as T3c. Using the 2002 staging system, cancer-specific survival at 5 years ranged from 97% for pT1a to 20% for pT4. Lymph node metastases portend a poor prognosis, with cancer-specific survival rates of 5% to 30% at 5 years and 0% to 5% at 10 years. Distant metastases to lung, bone, brain, or other organs are associated with survival rates of 50%, 5% to 30%, and 0% to 5% at 1, 5, and 10 years, respectively.
Prognostic factors
A criticism of staging systems is that they tend to provide limited prognostic ability, in part because there may be significant heterogeneity among patients in each classification, and often the systems do not take into account a number of significant predictive factors. This criticism has led investigators to explore various prognostic factors, either alone or in combination with the TNM staging system and/or other factors ( Box 1 ). Patients who present with either local or systemic symptoms have a worse prognosis than patients who have incidentally detected tumors. Moreover, the presence of symptoms of cachexia, including weight loss, anorexia, or malaise, or a reduction in overall health (Karnofsky scale or Eastern Cooperative Oncology Group [ECOG] performance status) at diagnosis confers a poor prognosis in both localized and metastatic RCC. Other prognostic factors in patients who have metastatic RCC include the number of metastases and site of metastases ( Table 1 ): patients who have a solitary lung metastasis account for almost all the complete remissions observed in clinical trials. Several laboratory values, including anemia (hemoglobin < 10 g/dL for females or < 12 g/dL for males), thrombocytosis, hypercalcemia, elevated alkaline phosphatase, elevated C-reactive protein, and erythrocyte sedimentation rate less than 30 mm/h, also portend poorer outcomes in patients who have RCC.
Anatomic
Tumor size
Extension into perinephric or renal sinus fat
Adrenal involvement (direct or metastatic)
Venous involvement
Lymph node metastases
Distant metastases
Metastatic burden of disease
Clinical
Performance status (Karnofsky, ECOG)
Localized symptoms
Systemic symptoms (cachexia, > 10-pound weight loss)
Thrombocytosis
Anemia
Hypercalcemia
Elevated alkaline phosphatase
Elevated C-reactive protein
Elevated erythrocyte sedimentation rate
Histologic
Nuclear grade
Histologic subtype
Presence of sarcomatoid features
Presence of histologic necrosis
Vascular invasion
Collecting system invasion
Molecular
Hypoxia-inducible factors: CA-IX, CA-XII, CXCR3, CXCR4, HIF, IGF-1, VEGF, VEGFRs
Co-stimulatory molecules: B7-H1, B7-H3 (tumor cell/vascular), B7-H4, PD-1
Cell cycle regulators: p53, Bcl-2, PTEN, Cyclin A, p27, Skp2
Adhesion molecules: EpCAM/KSA, EMA, E-Cad, alpha-catenin, Cad-6
Other factors: Ki-67, XIAP, Survivin, EphA2, Smac/DIABLO, PCNA, Caveolin-1, AR, CD44, Annexin II, Gelsolin, Vimentin, CA-125), aberrant DN A methylation, Na-K ATPase α1 subunit, vitamin D receptor, retinoid X receptor
Data from Lane BR, Kattan MW. Predicting outcomes in renal cell carcinoma. Curr Opin Urol 2005;15:289–97.
| Reference | Motzer 1999 | Mekhail 2005 | Motzer 2004 | Boumerhi 2003 | Escudier 2007 | Choueiri 2007 |
|---|---|---|---|---|---|---|
| N | 670 | 353 | 137 | 85 | 300 | 120 |
| Previous treatment | None | None | Immunotherapy and other therapies | Immunotherapy and other therapies | Immunotherapy | VEGF-targeted therapy |
| Conventional histology (%) | NA | 85 | 92 | 85 | 93 | 100 |
| Prior nephrectomy (%) | 65 | 81 | 74 | 85 | 94 | 100 |
| Median survival (months) | 10 | 14.8 | 12.7 | 16.5 | 12.6 | 13.8 a |
| Associated with adverse outcome? | ||||||
| Less than 1 to 2 years from nephrectomy or diagnosis to metastasis | Yes | Yes | No | No | Yes | Yes |
| Hemoglobin below lower limit of normal | Yes | Yes | Yes | Yes | No | No |
| Elevated alkaline phosphatase | No | No | No | Yes | Yes | No |
| Abnormal corrected calcium b | Yes | Yes | Yes | Yes | Yes | Yes |
| LDH more than 1.5 × upper limit of normal | Yes | Yes | No | No | Yes | No |
| Reduced performance status | Yes | No | Yes | No | No | Yes |
| No. metastatic sites | No | Yes | No | No | Yes | No |
| Prior radiotherapy | No | Yes | No | No | No | No |
| Low platelet count | No | No | No | No | No | Yes |
| Low neutrophil count | No | No | No | No | No | Yes |
b Corrected calcium = total calcium − 0.707 ∗ (albumin − 3.4).
Prognostic factors
A criticism of staging systems is that they tend to provide limited prognostic ability, in part because there may be significant heterogeneity among patients in each classification, and often the systems do not take into account a number of significant predictive factors. This criticism has led investigators to explore various prognostic factors, either alone or in combination with the TNM staging system and/or other factors ( Box 1 ). Patients who present with either local or systemic symptoms have a worse prognosis than patients who have incidentally detected tumors. Moreover, the presence of symptoms of cachexia, including weight loss, anorexia, or malaise, or a reduction in overall health (Karnofsky scale or Eastern Cooperative Oncology Group [ECOG] performance status) at diagnosis confers a poor prognosis in both localized and metastatic RCC. Other prognostic factors in patients who have metastatic RCC include the number of metastases and site of metastases ( Table 1 ): patients who have a solitary lung metastasis account for almost all the complete remissions observed in clinical trials. Several laboratory values, including anemia (hemoglobin < 10 g/dL for females or < 12 g/dL for males), thrombocytosis, hypercalcemia, elevated alkaline phosphatase, elevated C-reactive protein, and erythrocyte sedimentation rate less than 30 mm/h, also portend poorer outcomes in patients who have RCC.
Anatomic
Tumor size
Extension into perinephric or renal sinus fat
Adrenal involvement (direct or metastatic)
Venous involvement
Lymph node metastases
Distant metastases
Metastatic burden of disease
Clinical
Performance status (Karnofsky, ECOG)
Localized symptoms
Systemic symptoms (cachexia, > 10-pound weight loss)
Thrombocytosis
Anemia
Hypercalcemia
Elevated alkaline phosphatase
Elevated C-reactive protein
Elevated erythrocyte sedimentation rate
Histologic
Nuclear grade
Histologic subtype
Presence of sarcomatoid features
Presence of histologic necrosis
Vascular invasion
Collecting system invasion
Molecular
Hypoxia-inducible factors: CA-IX, CA-XII, CXCR3, CXCR4, HIF, IGF-1, VEGF, VEGFRs
Co-stimulatory molecules: B7-H1, B7-H3 (tumor cell/vascular), B7-H4, PD-1
Cell cycle regulators: p53, Bcl-2, PTEN, Cyclin A, p27, Skp2
Adhesion molecules: EpCAM/KSA, EMA, E-Cad, alpha-catenin, Cad-6
Other factors: Ki-67, XIAP, Survivin, EphA2, Smac/DIABLO, PCNA, Caveolin-1, AR, CD44, Annexin II, Gelsolin, Vimentin, CA-125), aberrant DN A methylation, Na-K ATPase α1 subunit, vitamin D receptor, retinoid X receptor
Data from Lane BR, Kattan MW. Predicting outcomes in renal cell carcinoma. Curr Opin Urol 2005;15:289–97.
| Reference | Motzer 1999 | Mekhail 2005 | Motzer 2004 | Boumerhi 2003 | Escudier 2007 | Choueiri 2007 |
|---|---|---|---|---|---|---|
| N | 670 | 353 | 137 | 85 | 300 | 120 |
| Previous treatment | None | None | Immunotherapy and other therapies | Immunotherapy and other therapies | Immunotherapy | VEGF-targeted therapy |
| Conventional histology (%) | NA | 85 | 92 | 85 | 93 | 100 |
| Prior nephrectomy (%) | 65 | 81 | 74 | 85 | 94 | 100 |
| Median survival (months) | 10 | 14.8 | 12.7 | 16.5 | 12.6 | 13.8 a |
| Associated with adverse outcome? | ||||||
| Less than 1 to 2 years from nephrectomy or diagnosis to metastasis | Yes | Yes | No | No | Yes | Yes |
| Hemoglobin below lower limit of normal | Yes | Yes | Yes | Yes | No | No |
| Elevated alkaline phosphatase | No | No | No | Yes | Yes | No |
| Abnormal corrected calcium b | Yes | Yes | Yes | Yes | Yes | Yes |
| LDH more than 1.5 × upper limit of normal | Yes | Yes | No | No | Yes | No |
| Reduced performance status | Yes | No | Yes | No | No | Yes |
| No. metastatic sites | No | Yes | No | No | Yes | No |
| Prior radiotherapy | No | Yes | No | No | No | No |
| Low platelet count | No | No | No | No | No | Yes |
| Low neutrophil count | No | No | No | No | No | Yes |
b Corrected calcium = total calcium − 0.707 ∗ (albumin − 3.4).
Histologic renal cell carcinoma subtypes
RCC is known to be a heterogeneous malignancy with several subtypes that exhibit distinct clinical and pathologic features. Several histologic subtypes of RCC have been described, including conventional clear cell RCC (cRCC), papillary RCC, and chromophobe RCC. Papillary RCC and chromophobe RCC account for about 15% to 25% of RCC, and patients who have these subtypes generally have a better long-term disease-free survival than those who have cRCC. Other rarer subtypes (collecting duct, medullary cell, and unclassified RCC) often display more aggressive clinical behavior. Consistent clinical behavior is not observed even within the major subtypes, however, suggesting that genetic heterogeneity exists within each subtype. The unique molecular defects that are pathogenic for each subtype are becoming defined, allowing targeted molecular approaches to be developed and tested and moving RCC to the forefront of molecular therapeutics. For example, the von Hippel-Lindau ( VHL ) gene on chromosome 3p25 has been implicated in von Hippel-Lindau disease, a condition in which patients often develop multiple, bilateral cRCC. Mutations in the VHL gene or hypermethylation of the VHL gene promoter region have been identified in 57% to 70% of patients who have sporadic RCC. A recent study, however, indicates that the presence of a detectable VHL mutation does not affect survival in patients who have localized cRCC. Dysregulation of hypoxia-inducible factors by alterations in VHL creates a vasculogenic environment favoring tumor growth. New agents targeting several signaling pathways that derive directly or indirectly from hypoxia-inducible factors, including VEGF, platelet-derived growth factor, epidermal growth factor receptor, and mammalian target of rapamycin, have proven effective in phase II and phase III clinical trials. Genes linked to the development of RCC also have been identified in individuals who have hereditary papillary RCC syndrome, Birt-Hogg-Dubé syndrome, and hereditary leiomyomatosis and RCC syndrome. Only 4% of patients who have RCC develop the disease within the context of these familial syndromes, however, suggesting that there are many other genes yet to be implicated in the pathogenesis of RCC. More recently, a number of molecular factors have been found to serve as independent prognostic factors for RCC.
Other pathologic factors
Pathologic factors, including Fuhrman nuclear grade and the presence of sarcomatoid features, provide prognostic information in patients who have localized RCC. Histologic tumor necrosis has been shown to be a negative prognostic indicator, as has collecting system invasion in low-stage lesions. Although the presence of multiple renal tumors may complicate surgical management, multifocality has been demonstrated to confer either a neutral or positive influence on outcomes. In addition, although involvement of the renal vein and inferior vena cava has a negative influence on prognosis, the impact of microvascular invasion on prognosis remains controversial.
Preoperative nomograms for suspected renal malignancy
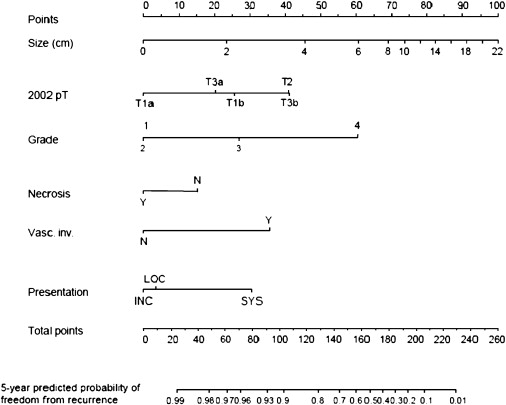
The authors recently have constructed a nomogram to predict the likelihood of benign or malignant pathology in patients who have a single enhancing renal neoplasm amenable to partial nephrectomy ( Fig. 1 ). Based on pathologic data obtained during 862 partial nephrectomies, 20% of suspected renal malignancies had benign histology. The predicted probability of benign disease ranged from 5% to 50% based on readily identifiable preoperative factors (tumor size, patient age and gender, symptoms at presentation, and smoking history). This information may be particularly applicable when active surveillance or tumor ablation is being considered for smaller tumors in more infirm patients. Several additional preoperative nomograms predicting recurrence-free survival have been developed, but none performs as well as algorithms that incorporate data obtained during nephrectomy. The authors believe that estimation of this end point is more useful in the postoperative setting, when this information might affect subsequent decision making (surveillance protocol, adjuvant treatment).
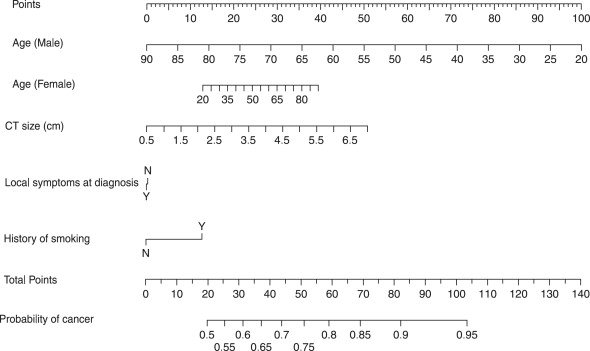
Postoperative prognostic algorithms for localized renal cell carcinoma
Several groups have developed postoperative prognostic algorithms based on clinical and pathologic features. In 2001, researchers at Memorial Sloan-Kettering Cancer Center proposed a nomogram for patients who have localized clear cell, papillary, or chromophobe RCC. Prognostic factors included tumor stage, tumor size, histologic subtype, and symptoms at presentation. Subsequently, the same group produced a revised nomogram for patients who had cRCC that was based on tumor stage, tumor size, nuclear grade, necrosis, vascular invasion, and symptoms at presentation ( Fig. 2 ). Such paper-based nomograms provide a visual aid clinicians can use during patient counseling. Software-based nomograms ( www.nomograms.org ) also provide accurate prognostic information and may take even less time to use during doctor–patient interactions.