Radical nephrectomy and regional lymphadenectomy have been the cornerstone of therapy for renal cell carcinoma for several decades; however, debate regarding the potential advantages of lymph node dissection for renal cell carcinoma continues. Currently, there are no definitive data indicating a survival advantage to lymphadenectomy, and systematic complete lymph node dissection adds time to the procedure and requires manipulation of the great vessels, which some surgeons may find challenging. This article examines the rationale for lymphadenectomy in the management of renal cell carcinoma and reviews the limited literature on the subject.
Radical nephrectomy and regional lymphadenectomy have been the cornerstone of therapy for renal cell carcinoma (RCC) for several decades; however, debate regarding the potential advantages of lymph node dissection for RCC continues. Currently, there are no definitive data indicating a survival advantage to lymphadenectomy. Furthermore, systematic complete lymph node dissection adds time to the procedure and requires manipulation of the great vessels, which some surgeons may find challenging. This article examines the rationale for lymphadenectomy in the management of renal cell carcinoma and reviews the limited literature on the subject.
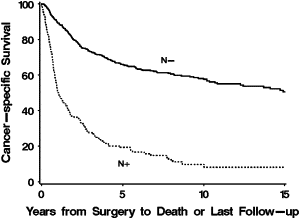
Carcinoma of the kidney and renal pelvis is expected to be newly diagnosed in over 54,000 patients in the United States and will result in over 13,000 deaths, accounting for approximately 3% of all cancer deaths in 2008. About one-third of new patients presenting with RCC have metastatic disease. Another third of patients presenting with localized disease eventually experience recurrence and progression. Approximately 25% of patients with metastatic RCC have clinically evident lymphadenopathy. While metastatic disease is highly resistant to chemotherapy, systemic therapy options now include targeted therapy in addition to immunotherapy. Thankfully, survival for patients with RCC appears to be improving, with decreasing death rates per 100,000 from 6.16 to 5.91 in men and from 2.95 to 2.72 in women between the early 1990s and today. This is reflected in improved 5-year survival rates from 52% between 1974 and 1976 to 63% in 1999. Positive nodes have been clearly shown to have an independent adverse effect on outcome, regardless of other prognostic factors. For patients with node-positive disease, 5-year survival rates range between 5% and 35%. Most studies of node-positive renal cell carcinoma report 5-year survival rates of about 15%. Fig. 1 demonstrates the impact of lymph node status on cancer-specific survival among patients treated surgically for RCC at the Mayo Clinic.
Proponents of lymphadenectomy point to higher survival rates for patients undergoing radical nephrectomy plus extended lymph node dissection, compared with historical studies that did not include routine lymphadenectomy. Opponents point to the high rates of hematogenous metastases and question the value of lymph node dissection in a disease that follows an unpredictable course. To definitively address the potential benefit of lymphadenectomy in RCC, the European Organization for Research and Treatment of Cancer Genitourinary Group launched a head-to-head randomized phase 3 trial at multiple European centers in 1988. The trial, which completed enrollment in 1992, is designed to compare the long-term results of radical nephrectomy with complete lymphadenectomy ( n = 383) against radical nephrectomy alone ( n = 389) in patients without evidence of metastases. Early results indicate that complete lymph node dissection did not increase morbidity associated with radical nephrectomy. Pathology confirmed lymph node metastases in 3.3% of clinically negative nodes after lymphadenectomy. However, survival in the study so far had been reported to be excellent overall and more follow-up time is needed to compare tumor-free survival and overall survival between the lymphadenectomy and non-lymphadenectomy groups. While results are pending, debate will continue regarding the need for lymphadenectomy in RCC patients.
Although the role of nephron-sparing surgery for renal masses under 7 cm continues to evolve, the appropriate surgical treatment for large renal masses has not changed substantively since Robson and colleagues first reported increased survival in a small cohort of patients who received lymphadenectomy in 1969. Surgical excision of solid tumors was common by the late nineteenth century. After Halsted demonstrated the efficacy of extensive regional lymphadenectomy for breast cancer in 1894, radical excision and regional lymphadenectomy gradually evolved as the standard of care for most carcinomas. During the first half of the twentieth century simple nephrectomy became the standard treatment for localized RCC. The procedure typically involved removal of the kidney from the surrounding Gerota’s fascia. Surgeons generally left the perirenal fat, adrenal gland, and regional lymph nodes in situ.
The first radical nephrectomy, removing the kidney, adrenal gland, and the surrounding Gerota’s fascia, was reported by Mortensen in 1948. The reported rationale for the extent of surgery was the observation that pathology studies revealed perirenal fat infiltration in 13% of renal tumors; therefore, removal of the fat and the organs contained within was postulated to increase survival. In the 1960s, Robson and colleagues added retroperitoneal lymphadenectomy to radical nephrectomy and reported improved 5-year survival rates.
The first systematic survey of lymphatic drainage from the kidneys was published in 1935, when Parker reported on the dissection of cadavers and stillborn infants to detail the renal lymphatic system. For the right kidney, the first-echelon nodes are the precaval, retrocaval, and interaortocaval lymph nodes. The paracaval nodes are not a primary drainage route for the right kidney. For the left kidney, the primary drainage nodes include the para-aortic, preaortic, and retroaortic lymph nodes. By the early 1990s other groups had confirmed Parker’s findings by mapping the sites of involved nodes in node-positive patients who underwent extended lymph node dissection. Right kidney metastases were most commonly found in the interaortocaval and retrocaval area. The hilar and paracaval nodes were less frequently involved. Most left kidney metastases were found in the hilar, para-aortic, and retroaortic areas. Isolated metastases were also found in the ipsilateral iliac nodes and in the supraclavicular nodes.
A hilar lymphadenectomy removes only the lymph nodes surrounding the renal vessels, while a regional dissection typically includes the para-aortic region on the left and the paracaval area on the right. Such a procedure may be adequate for tumors of the left kidney, but would miss the most common site for lymphatic metastases from the right kidney, the interaortocaval area. If the urologist wishes to minimize the risk of leaving nodal metastases in situ, the authors advocate an extended lymphadenectomy. On the left side, an extended dissection includes removal of the preaortic, para-aortic, retroaortic, interaortocaval, and precaval lymphatic tissue from the diaphragm to the bifurcation of the aorta. On the right side, extended dissection includes removal of the precaval, paracaval, retrocaval, interaortocaval, and preaortic lymph nodes.
There are only two reasons to perform an extended lymphadenectomy in conjunction with a radical nephrectomy: one is to improve staging accuracy. Although modern imaging techniques, such as computed tomography, magnetic resonance imaging, ultrasound, and positron emission tomography offer excellent diagnostic and staging, none are able to reliably predict nodal involvement. In terms of staging RCC, the overall accuracy of CT scanning, generally accepted as the most sensitive and accurate imaging method for staging, ranges from 61% to 91%. Future advances in imaging, such as the use of lymphotrophic ferromagnetic nanoparticles in conjunction with MRI, may allow detection of lymphatic metastases in patients without overt lymphadenopathy. Perhaps a more debatable rationale for lymphadenectomy is to improve patient outcomes by removing overt or subclinical lymph node metastases. It is possible that lymphadenectomy may be beneficial because of other mechanisms, such as removal of potential immunosuppressive factors in antigen-primed lymph nodes in the absence of metastases.
Terrone and colleagues recently examined the relationship between the accuracy of tumor staging and the number of nodes examined by retrospectively reviewing the number of lymph nodes removed during radical nephrectomy for RCC in 725 patients. Of the 725 cases, 608 (84%) had lymph nodes removed. Of the 608 patients with nodes identified pathologically, 13.6% had lymphatic metastases. Interestingly, the rate of positive lymph nodes was higher if more than 12 nodes were found with 21% positive nodes in patients with greater than or equal to 13 nodes and 10% positive for patients with fewer than 13 nodes in the specimen. The investigators concluded that lymphadenectomy is essential to properly stage a tumor, regardless of the potential therapeutic impact. With the recent introduction of several targeted therapies for RCC, and in the setting of ongoing clinical trials of adjuvant therapy for high risk resected RCC without hematogenous metastases, accurate staging will become increasingly important.
The radiographic finding of enlarged lymph nodes in patients with renal masses has been shown to have poor correlation with pathology. Studer and colleagues reviewed the CT scans of 163 patients with RCC. CT scans were falsely negative in five patients. Furthermore, among 43 patients with retroperitoneal lymphadenopathy by CT criteria (lymph nodes 1 cm–2.2 cm), only 18 (42%) had lymph node metastases. The 58% of patients with lymphadenopathy by CT criteria and no pathologically confirmed lymph node metastases were found to have only inflammatory changes or follicular hyperplasia. Lymphadenopathy on CT because of inflammatory changes or follicular hyperplasia was more common among patients with venous tumor thrombus or tumor necrosis. This study further supports the need for extended lymphadenectomy in patients where accurate staging is important.
Modern evidence for the potential therapeutic benefit of lymphadenectomy in conjunction with radical nephrectomy is mounting. In 1991 Herrlinger and colleagues reported a significant survival advantage in a study of 511 patients who were treated with extended lymphadenectomy with radical nephrectomy, compared with those whose lymph nodes were removed only if clinically abnormal or for staging purposes (the investigators referred to this group as “facultative lymphadenectomy”). In patients with left kidney tumors, extended lymphadenectomy involved the removal of all preaortic, para-aortic, and retroaortic lymph nodes from the diaphragm to the bifurcation of the aorta. For right kidney tumors, all paracaval, precaval, retrocaval, and interaortocaval nodes were removed from the diaphragm to the bifurcation of the vena cava. Operative mortality was 1% in the extended lymphadenectomy group and 3.8% in the facultative lymphadenectomy group.
Herrlinger and colleagues reported substantial differences in the rate of node-positive disease and outcome between the two surgical groups. In the extended lymphadenectomy group, pathologists found 17 or more nodes removed with each radical nephrectomy. In the facultative lymphadenectomy group, more than half of patients had no nodes removed, one to five nodes were removed in 30% of patients, and more than five nodes in 10% of patients. The incidence of positive nodes in the lymphadenectomy group was 17.5% versus 10% for the nonlymphadenectomy group. There was a significant difference in survival between the two treatment groups, with 5- and 10-year survivals of 66% and 56.1%, compared with 58% and 40.9% for the extended lymphadenectomy and facultative lymphadenectomy groups, respectively. The authors reported that the survival advantage was more pronounced for patients with low-stage tumors. In patients with organ-confined RCC, extended lymphadenectomy produced a 5-year survival rate of 91.6% and a 10-year survival rate of 80.2%, compared with 81.3% and 54% for patients who did not receive the extended lymph node dissection. For patients with localized RCC who had extra-renal disease, survival was 76% at 5 years and 58.2% at 10 years, compared with 54.5% and 41.2% without lymphadenectomy. There was no statistically significant difference in survival for more advanced patients. Herrlinger and colleagues concluded that there could be no doubt that extended lymphadenectomy improves survival for patients with low-stage disease. The improvement, they concluded, is a result of the removal of subclinical microscopic metastases in the regional lymph nodes that would otherwise have resulted in dissemination of disease after radical nephrectomy alone. Assuming that patients are reasonable candidates for curative surgery, the group concluded that there is no preoperative or intraoperative staging procedure that could effectively define any group of patients who would not benefit from extended lymphadenectomy.
Phillips and Messing retrospectively reviewed the impact of lymphadenectomy with regard to the subsequent development of local recurrence of RCC following radical nephrectomy in 1993. Patients with nodal metastases who received radical nephrectomy without lymphadenectomy had a local recurrence rate of 86%. None of the patients with node-positive disease who received radical nephrectomy plus lymphadenectomy experienced a local recurrence. There was no increase in morbidity of the surgery as a result of lymphadenectomy.
Schafhauser and colleagues conducted a retrospective review of 1,035 patients with RCC treated between 1974 and 1993. Patients were classified based on the extent of the lymphadenectomy into three groups: group A had a systematic extended lymphadenectomy, group B had only grossly abnormal nodes resected, and group C had no lymph nodes resected. Group A patients had the larger tumors with higher stage and grade RCC relative to groups B and C. Despite the fact that patients in group A had more adverse tumor characteristics, the highest mean survival rate at 5 years (70.1% compared with 61.8% and 65.6%) and 10 years (58.3% compared with 50.4% and 44.5%) was seen in the group A patients. The investigators concluded that radical nephrectomy plus extended lymphadenectomy benefits at least 4% of all patients.
More recently, Pantuck and colleagues retrospectively reviewed 900 patients treated surgically for RCC. The study population was comprised of a high proportion of patients with clinically evident adenopathy and advanced RCC. The investigators found that lymphadenectomy offered no benefit in terms of survival or recurrence of disease in patients with clinically negative lymph nodes. The investigators concluded that the lack of demonstrable benefit in this study was likely a result of the low incidence of positive nodes discovered based on pathology alone. Fewer than 8% of node-positive cases in Pantuck’s study were discovered upon pathology after resection. The other 92% of positive nodes had been identified either pre- or intraoperatively. However, among patients with lymph node-positive RCC a distinct advantage in survival was demonstrated for those who underwent lymphadenectomy versus those without lymphadenectomy. Furthermore, patients treated with lymphadenectomy and subsequent interleukin-2 based immunotherapy had a trend toward improved response to systemic therapy. The study concluded that when positive nodes are found, they should be resected when technically feasible.
Recently Minervini and colleagues retrospectively reviewed 167 radical nephrectomy cases and found no difference in survival between 108 patients with radical nephrectomy alone and 59 patients with regional lymphadenectomy. Regional lymphadenectomy was defined as removal of the lymph nodes anterior, posterior, and lateral to the ipsilateral great vessel from the renal pedicle to the level of the inferior mesenteric artery. Among the 59 patients that had a regional lymphadenectomy, 10 had clinically evident lymphadenopathy. Only 1 of 49 patients without lymphadenopathy had pathologically involved lymph nodes. The investigators conclude that in the absence of lymphadenopathy there is no benefit to lymphadenectomy.
Canfield and colleagues recently reviewed the records of 40 patients treated with radical nephrectomy and regional lymphadenectomy (excluding the interaortocaval lymph nodes), all of whom had positive lymph nodes and no evidence of hematogeneous metastases. Median cancer-specific survival was 20 months, and with a median follow-up of 17.7 months 30% of patients remained free of RCC. The investigators suggest that aggressive resection of lymph node metastases may impart a survival advantage based on these findings. The authors have recently reviewed their experience with resection of isolated metachronous recurrence of RCC in the retroperitoneal lymph nodes. Fifteen patients who underwent surgical resection of isolated, metachronous recurrence of RCC in the retroperitoneal lymph nodes following radical nephrectomy were compared with patients who had lymph node-positive RCC at the time of nephrectomy and to patients who underwent complete resection of a solitary metachronous metastasis of RCC at another site. No intra- or postoperative mortality occurred. Six of the 15 patients that underwent resection of metachronous lymph node metastasis subsequently died from RCC at a median of 18 months (range 6–33.6) following resection. The median cause-specific survival was 33.3 months for patients with metachronous lymph node metastases versus 20.8 months and 46.9 months for patients with synchronous lymph node metastases and metachronous nonlymph node metastases, respectively.
It is clear that without prospective studies of standardized lymph node resection, no survival advantage can be proven based on the varied retrospective data to date. The question, then, is how to identify the small (3%–8%) cohort of patients with unidentified positive nodes for whom extended lymphadenectomy is most likely to be useful and which nodes should be removed. The authors have recently analyzed more than 1,600 patients with clear cell RCC from the Mayo Clinic Nephrectomy Registry. The study identified five features that were associated with lymph node metastases: primary tumor stage T3 or T4, nuclear grade 3 or 4, tumor size 10 cm or greater, presence of a sarcomatoid component, and presence of histologic tumor necrosis.
Patients with none of the risk features or any one feature have a low likelihood of regional lymph node involvement (0.6%). But patients exhibiting any two of the five features have a 4.4% risk of regional lymph node involvement and patients with all five features have a greater than 50% risk of lymph node-positive disease. The authors therefore suggest that lymphadenectomy is indicated for patients with any two or more risk features, which can be determined by frozen section analysis intraoperatively.
In summary, there are adequate data to suggest that patients can benefit from resection of clinically evident lymphadenopathy at the time of radical nephrectomy. There are currently insufficient data regarding patients with clinically negative retroperitoneal lymph nodes to mandate lymphadenectomy at the time of radical nephrectomy. The authors’ bias is that patients with large tumors, high grade or stage, or other adverse features should have a lymphadenectomy, and that more extensive lymph node resection will be more likely to confer maximum benefits. For right side tumors, lymphadenectomy should include dissection of the vena cava from the diaphragm to the bifurcation of the vessels, including the precaval, paracaval, retrocaval, and interaortocaval nodes. For left side tumors, dissection should include the preaortic, para-aortic, and retroaortic nodes from diaphragm to the bifurcation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








