Pregnancy-Induced Anatomic and Physiologic Changes
Kidney size increases by approximately 1 cm during pregnancy. The urinary collecting system (renal calyces, pelvis, and ureters) dilates. Hormonal and mechanical forces are thought to account for ureteral dilation as early as 6 weeks gestation. In the later stages of pregnancy, mechanical compression of the ureter against the pelvic brim may lead to hydroureter and hydronephrosis. Hydronephrosis occurs on the right in 90% of cases due to dextrorotation of the uterus by the sigmoid colon.
In rare instances this becomes a clinically significant cause of obstructive uropathy. The dilated collecting systems can hold up to 300 mL of urine and hence serve as a reservoir for bacteria. The dilated urinary tract also allows for urinary stasis and increases the risk of pyelonephritis in pregnant women with asymptomatic bacteriuria.
Renal physiologic changes are characterized by marked vasodilation, which leads to increases in glomerular filtration rate (GFR) and renal plasma flow (RPF). These changes occur early in the first trimester and peak increases in GFR and RPF to 50% above baseline are seen by the end of the first trimester. The filtration fraction (GFR/RPF) falls significantly, indicating a greater rise in effective RPF. Creatinine production is unchanged in pregnancy but creatinine clearance is increased, resulting in lower levels of serum creatinine; the normal creatinine value during pregnancy is <0.8 mg/dL (see Table 55–1).
Blood urea nitrogen (BUN), mg/dL | 7–10 |
Creatinine, mg/dL | 0.3–0.6 |
Creatinine clearance, mL/minute | 150–200 |
Uric acid, mg/dL | 3.2–4 |
Sodium, mEq/L | 130–135 |
24-hour urine protein, mg | <300 |
Arterial pH | 7.4–7.45 |
Pco2, mm Hg | 27–32 |
HCO3, mEq/L | 18–21 |
Increased urinary excretion of protein, amino acids, uric acid, glucose, and calcium occurs as a result of the elevated GFR. Hence, proteinuria in pregnancy is considered abnormal when it exceeds 300 mg/day compared with an upper limit of normal of 150 mg/day in the nonpregnant population. Uric acid clearance also increases in pregnancy and serum uric acid levels in pregnant women usually do not exceed 4.5 mg/dL by the third trimester.
Pregnancy is associated with significant changes in water metabolism. Serum osmolality falls by 5–10 mOsmol/kg as a result of several forces. The serum osmostat is reset as suggested by normal responses to water loading and water deprivation despite a lower serum osmolality. There is a decrease in the osmotic thresholds for thirst and arginine vasopressin (AVP) release. Enhanced catabolism of AVP by release of placental vasopressinases leads to transient diabetes insipidus and in some women is severe enough to warrant treatment. Total body water increases by 6–8 L, most of which is extracellular. Plasma volume increases throughout pregnancy by 1.1–1.6 L, resulting in a plasma volume of 4.7–5.2 L, 30–50% above that in nonpregnant women. This is accompanied by the retention of 900–1000 mEq of sodium, which contributes to the mild edema seen in some pregnant women. Red blood cell mass increases 20–30% above baseline by the end of pregnancy. The proportionally greater increase in intravascular volume relative to red cell mass results in the dilutional or physiologic anemia of pregnancy.
Serum sodium falls by 5 mEq/L due to resetting of the osmostat (Table 55–1). Hyponatremia during pregnancy parallels the increased release of human chorionic gonadotropin (hCG), which appears to mediate these changes via the release of relaxin. Serum potassium levels are normal despite increased serum aldosterone, perhaps due to the potassium-sparing effects of elevated progesterone levels in pregnancy. Elevated progesterone levels stimulate hyperventilation and cause mild respiratory alkalosis, resulting in a slight increase in arterial pH and a fall in plasma bicarbonate concentrations by about 4 mEq/L. Total serum calcium levels fall in pregnancy but ionized calcium remains normal. Accelerated renal and placental production of calcitriol leads to increased gastrointestinal absorption of calcium and absorptive hypercalciuria with urine calcium as high as 300 mg/day. Serum parathyroid hormone (PTH) concentrations are lower than normal, partly in response to higher serum levels of calcitriol.
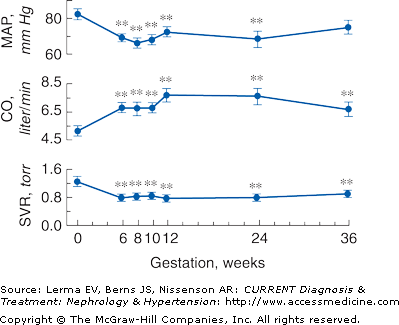
Profound changes in cardiovascular physiology and systemic hemodynamics are required to accommodate the increased metabolic demand of normal pregnancy. Pregnancy is accompanied by a rise in cardiac output and fall in mean arterial blood pressure and systemic vascular resistance (Figure 55–1). Cardiac output increases by 30–50% (1.8 L/minute) above prepregnancy values. Increased preload (due to increased blood volume), decreased afterload (due to decreased systemic vascular resistance), and the increase in maternal heart rate account for the rise in cardiac output. Cardiac output in pregnant women is affected significantly by changes in posture that compromise preload by compression of the inferior vena cava by a gravid uterus.
Figure 55–1.
Systemic hemodynamic changes throughout early human pregnancy. Mean arterial pressure (MAP) and cardiac output (CO) increase significantly in early gestation in association with a fall in systemic vascular resistance (SVR). (Modified with permission from Chapman AB et al: Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 1998;54:2056.)
The fall in systemic vascular resistance (SVR) is manifested by a fall in blood pressure, which begins by the end of the first trimester. Blood pressure falls to 10 mm Hg below baseline by the second trimester, declining to a mean of 105/60 mm Hg. In the third trimester, the diastolic blood pressure gradually increases to nonpregnant values by term. Mechanisms underlying the fall in SVR and subsequent vasodilation include decreased vasopressor responsiveness to angiotensin II and norepinephrine, enhanced production of the vasodilatory factors prostacyclin and nitric oxide, and decreased aortic stiffness.
The renin–angiotensin–aldosterone system (RAS) is highly activated in normal human pregnancies. Plasma renin activity (PRA) increases 4-fold in the first trimester and continues to rise until approximately 20 weeks gestation. The increase in PRA stimulates increased secretion of aldosterone. The activation of the RAS is thought to be secondary, in response to vasodilation and a decrease in blood pressure.
Effect of Kidney Disease on Pregnancy
Table 55–2 summarizes the two issues involved in chronic kidney disease/end-stage renal disease (ESRD) and pregnancy: the effects of kidney disease on pregnancy (course, complications, and outcome) and the effects of pregnancy on kidney disease.
Effects of pregnancy on kidney disease | Effects of kidney disease on pregnancy |
|---|---|
Worsening proteinuria | Infertility |
Loss of kidney function | Preterm delivery |
Hypertension and preeclampsia | IUGR |
Decreased fetal survival | |
Preeclampsia |
Pregnancy is not common in women on dialysis. Fertility rates in patients with ESRD are extremely low and are difficult to quantify accurately. Chronic kidney disease (CKD) is also associated with infertility.
The reproductive hormonal milieu in patients with ESRD remains an enigma. Elevated levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and low levels of free testosterone are common in men with ESRD.
Women with ESRD are usually amenorrheic or have irregular menstrual cycles. The frequency of menstruation in women of childbearing age with ESRD is variable, between 10% and 42%. Anovulatory cycles commonly occur even among menstruating women with ESRD. In contrast to women with ESRD, premenopausal women with normal kidney function have both tonic and cyclic components of gonadotropin secretion. The tonic component is regulated through basal gonadotropin secretion; the cyclic component is regulated through the midcycle surge of LH and its effects on the hypothalamus. The ovarian dysfunction in women on dialysis is characterized by the absence of cyclic gonadotropin release, presumably hypothalamic in origin. Menopause tends to occur earlier among women on dialysis.
Prolactin levels are elevated in both men and women on dialysis due to increased production and reduced renal clearance. Strikingly high numbers of women on hemodialysis (70–90%) and most peritoneal dialysis patients have hyperprolactinemia. Because of all of these factors, successful conception in dialysis patients is rare. Pregnancy is more common in kidney transplant recipients and in women with chronic kidney disease.
The difficulties of conception notwithstanding, successful pregnancies in dialysis-dependent patients are rare: of 115 pregnancies reported by the European Dialysis and Transplant Association, 23% carried to term. Fetal outcomes have improved over time: the percentage of pregnancies with surviving infants reported from the United States was 21% prior to 1990 and 52% from 1990 to 1991. The U.S. registry of pregnancy in dialysis patients reported that 42% of pregnancies resulted in a surviving infant, 7.5% in neonatal death, 6% in still birth, 32% in spontaneous abortion, and 10.5% in therapeutic abortion. Only 12% of the therapeutic abortions were performed due to pregnancy complications. Women who started dialysis during their pregnancy, conceived close to the point of requiring dialysis, or experienced a rapid decline in kidney function during their pregnancy have fairly good infant survival (73.6%) compared to that in women who conceived after starting dialysis (40% infant survival).
Prematurity and low-birth-weight babies are, unfortunately, the norm. Most deliveries in women who conceive on dialysis are premature (mean gestational age 32.4 weeks) and 36% of these infants weighed less than 1500 g at birth. Long-term medical and developmental problems are also common in these children and are due to prematurity and low birth weights rather than the azotemic intrauterine environment.
Polyhydramnios, which can precipitate preterm labor and postpartum infant complications, is an important fetal complication in pregnancies in women with CKD. It is thought to be the result of increased fetal diuresis in response to high maternal blood urea nitrogen levels. The resulting fetal azotemia resolves rapidly after delivery but can lead to volume depletion and electrolyte imbalance from the ensuing osmotic diuresis.
Obstetric management of these infants involves preventing preterm labor and appropriate fetal surveillance and timing of delivery. Strategies to prevent preterm labor include cautious use of magnesium or indomethacin in women with polyhydramnios. Magnesium levels must be closely monitored to avoid toxicity and consequent respiratory distress. Indomethacin can result in the loss of residual renal function and hyperkalemia, necessitating the initiation of dialysis or an increase in dialysis dose. Mid trimester losses may be prevented by monitoring dialysis patients for cervical shortening or cervical incompetence.
The timing of delivery is controversial. In most dialysis patients, preterm labor or an obstetric complication necessitates early delivery. Some obstetricians prefer delivery at 34–36 weeks if fetal lung maturity can be demonstrated. In transplant recipients and those with CKD, delivery is delayed until the onset of labor as long as maternal and fetal conditions remain optimal. A multidisciplinary team of health care providers including nephrologists, obstetricians, perinatologists, and dialysis providers is needed to ensure optimal care for the pregnant woman with CKD or ESRD.
Effects of Pregnancy on Kidney Disease
Pregnancy may influence the course of kidney disease. Importantly, women with CKD do not have the normal pregnancy-associated increase in GFR. Overall, the pregnancy outcome is usually favorable in women with mild CKD, serum creatinine less than 1.4 mg/dL, and normal blood pressure early in pregnancy. Several case series suggest that women who conceive with a serum creatinine greater than 1.4 mg/dL experience a more rapid deterioration in kidney function than their nonpregnant counterparts with similar degrees of kidney function. In a study of 82 pregnancies in 67 women with a serum creatinine level of 1.4 mg/dL or greater at conception or in their first trimester, 20% of women had a decline in kidney function during pregnancy and 23% had a decline by the first 6 weeks postpartum. At 6 months postpartum, 8% of women recovered kidney function to their baseline values and an additional 10% had deterioration in kidney function. Women with a baseline creatinine exceeding 2.0 mg/dL experienced the greatest decrease in kidney function: 10% of this group had a rapid loss of kidney function and progressed to ESRD within 1 year of delivery. Women with pregnancy-associated deterioration in kidney function account for approximately 20% of women requiring dialysis in pregnancy. Obstetric complications include a high rate of preterm delivery (59%) and fetal growth retardation (37%). The infant survival rate was 93%.
Proteinuria usually increases in pregnant women with CKD. Hypertension occurs or is exacerbated in most women with underlying kidney disease and usually requires antihypertensive medications. The Registry of Pregnancy in Dialysis Patients reported that 79% of pregnant patients with ESRD were hypertensive and 48% experienced a blood pressure greater than 170/110 mm Hg during their pregnancy. In women with moderate to severe CKD, the frequency of hypertension rose from 28% at baseline to 48% in the third trimester. Maternal mortality, although rare, has certainly been reported.
Kidney disease that develops during pregnancy is usually due to new onset glomerulonephritis, lupus nephritis, interstitial nephritis, or acute renal failure. The evaluation of kidney disease during pregnancy includes the measurement of kidney function, serologic testing, and ultrasonography. Renal biopsy is rarely employed and is reserved for unexplained deterioration in renal function or severe nephritic syndrome. There is no role for a renal biopsy in pregnancy beyond 32 weeks.
Pregnancy in women with diabetes mellitus is relatively common. The presence of diabetic nephropathy (urine albumin excretion >300 mg/day) at conception is a significant risk factor for perinatal morbidity and mortality. Preeclampsia superimposed upon diabetic nephropathy enhances the risk for preterm delivery and intrauterine growth retardation.
Among women with gestational diabetes, the prevalence of preeclampsia is 10–20%. The frequency of preeclampsia is higher with increasing severity of diabetes and in those with proteinuria at conception. Preterm delivery may be as high as 30% in women with preeclampsia. Diabetic women with microalbuminuria (urine albumin excretion of 30–300 mg/day) are also likely to develop preeclampsia, a risk similar to that of women with diabetic nephropathy. Preeclampsia also substantially increases the prevalence of their preterm delivery.
Early reports suggested pregnancy had little effect on the long-term progression of diabetic nephropathy, but recent reports contradict this. The progression of kidney disease is accelerated in 45% of pregnant women with diabetic nephropathy. A significant elevation in maternal blood pressure and proteinuria with nephrotic syndrome develops in most (71%) of these pregnancies. Infant birth weight correlates with gestational age and maternal kidney function: 71% of infants are appropriate for gestational age, 16% are small for gestational age, and 13% are large for gestational age. A prepregnancy history of tight glycemic control, a urine albumin excretion of less than 500 mg/day, and angiotensin-converting enzyme inhibitor (ACEI) therapy have been linked to a prolonged protective effect on maternal kidney function and more favorable pregnancy outcomes.
Systemic lupus erythematosus (SLE) is a disease often seen in women of childbearing age. Signs of active SLE early in pregnancy herald a hazardous course. As with other nephropathies, the presence of hypertension and elevated creatinine level increases the risk of complications. Antiphospholipid syndrome (APS) can be primary or secondary. Secondary APS is associated with SLE, other collagen vascular diseases, and malignancy. APS is associated with arterial and/or venous thrombosis and recurrent fetal loss, particularly in the second trimester, and is usually accompanied by mild to moderate thrombocytopenia and elevated titers of antiphospholipid antibodies (aPLs). aPLs, either anticardiolipin antibodies of the IgG or IgM serotype or the lupus anticoagulant, link the seemingly disparate syndromes of recurrent pregnancy loss with spontaneous thrombosis and suggest that the placenta and systemic blood vessels may in some way be targets of autoantibodies with similar phospholipid specificity. Preliminary classification criteria for APS are described in Table 55–3. This classification of pregnancy loss recognizes that a preterm live birth accompanied by severe preeclampsia or severe placental insufficiency is comparable to fetal loss late in pregnancy.
Clinical criteria |
Vascular thrombosis |
1. One or more clinical episodes of arterial, venous, or small-vessel thrombosis in any tissue or organ. |
2. Thrombosis confirmed by imaging or Doppler studies or histopathology, with the exception of superficial venous thrombosis. |
3. For histopathologic confirmation, thrombosis should be present without significant evidence of inflammation in the vessel wall. |
Pregnancy morbidity |
1. One or more unexplained deaths of a morphologically normal fetus at or beyond the tenth week of gestation, with normal fetal morphology documented by ultrasound or by direct examination of the fetus. |
2. Or one or more premature births of a morphologically normal neonate at or before week 34 of gestation because of severe preeclampsia or severe placental insufficiency. |
3. Or three or more unexplained consecutive spontaneous abortions before the tenth week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded. |
Laboratory criteria |
1. Anticardiolipin antibody of IgG and/or IgM isotype in blood, present in medium or high titer, on at least two occasions at least 6 weeks apart, measured by standard enzyme-linked immunosorbent assay for β2-glycoprotein I-dependent anticardiolipin antibodies. |
2. Or lupus anticoagulant present in plasma, on two or more occasions at least 6 weeks apart, detected according to the guidelines of the International Society on Thrombosis and Hemostasis. |
Preconception counseling and close maternal–fetal monitoring are recommended during all pregnancies of aPL-positive women. Recommendations for pharmacologic treatment are controversial and specific to a woman’s clinical and obstetric history. Combination aspirin and heparin therapy has been recommended. Warfarin is contraindicated during pregnancy (Table 55–4) but can be used in the postpartum period. Both heparin and warfarin are safe for nursing mothers. Primary aPL syndrome and aPL syndrome with SLE are treated similarly.
Medication | Safety issues | Comments |
|---|---|---|
Common medications in CKD/ESRD | ||
1. Erythropoietin | Safe to use | Limited data |
2. Iron | Safe to use | Low dose intravenous iron recommended |
3. Vitamin D | Widely used | Limited data |
4. Heparin | Safe to use | Minimize dose of heparin |
5. Coumadin | Not safe | Teratogenic |
Common medications in kidney transplantation | ||
1. Prednisone | Safe to use | Fetal adrenal insufficiency |
2. Cyclosporine | Safe to use | IUGR |
3. Tacrolimus | Not safe to use | Severe IUGR, renal failure, and hyperkalemia |
4. Mycophenolate mofetil | Not safe to use | Teratogenic in animals |
5. Azathioprine | Widely used |