General Considerations
The major forces responsible for solute transport across the membrane are diffusion and convection. Diffusion is influenced by the concentration gradient of the solute, the solute characteristics (eg, molecular weight and charge), and the membrane characteristics (eg, pore size and number). Removal of solutes by diffusion is enhanced by a large concentration gradient, small solute size, and a membrane with a large surface area and many large pores. The concentration gradient is maximized by using countercurrent flow of blood and dialysate.
In convection, hydrostatic or osmotic pressure forces water across the membrane. The water transport facilitates the passage of solutes across the membrane. The term ultrafiltration describes the solute and fluid removal via convection.
In hemodialysis, the predominant mechanism for solute removal is through diffusion, with a smaller amount of solute clearance occurring by convection. Thus, hemodialysis is very effective in removing solutes of small-molecular-weight, but is relatively inefficient in removing solutes of larger sizes. In addition, hemodialysis is an inefficient means of removal of protein-bound substances. Only the free portion of these solutes can diffuse across the membrane and be removed. The removal rate of protein-bound compounds thus depends on the concentration of the unbound solute, the size of the protein, and the replacement rate of the unbound solute.
Treatment
Patients should be considered for initiation of chronic hemodialysis therapy once the estimated glomerular filtration rate (GFR) is less than 15 mL/minute. In most patients, the four variables in the Modification of Diet in Renal Disease (MDRD) equation can be used to estimate the GFR. A 24-hour urine collection for creatinine and urea should be considered in those patients who have reduced muscle mass due to medical conditions such as amputations or limitation on mobility due to congestive heart failure, claudication, chronic lung disease requiring oxygen therapy, etc. There are no randomized trials that suggest an optimal time to initiate chronic dialysis therapy, so clinical judgment is important in making this decision in individual patients.
There are specific indications for starting chronic hemodialysis therapy at a level above a GFR of 15 mL/minute. These conditions include intractable fluid overload not responsive to diuretics, hyperkalemia unresponsive to medical therapy, metabolic acidosis not fully corrected by medical therapy, malnutrition or weight loss not ascribed to other medical conditions, or decreasing functional status. It may also be desirable to start home dialysis therapies at a higher level of GFR to minimize training difficulties due to neurologic dysfunction at lower levels of GFR.
Patients can be considered for a later initiation of dialysis if they are asymptomatic from a uremic standpoint, have adequate nutritional status, and do not have a decline in either dry weight or serum albumin levels. If renal replacement therapy is delayed, then the patient should be reassessed on a regular basis for a change in these parameters.
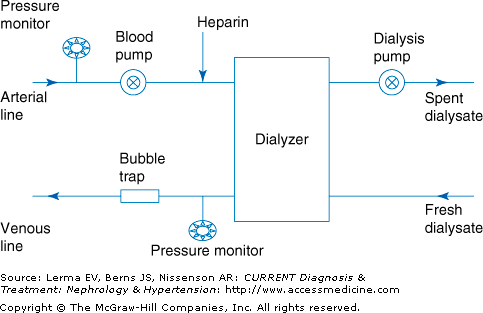
The major parts of the dialysis machine are the blood pump, dialyzer, dialysate pump, safety monitors, and alarms (Figure 50–1).
The artificial kidney or dialyzer consists of the blood compartment, the dialysate compartment, and the semipermeable membrane. The surface area of the dialyzer membrane can be increased by using either parallel plates or hollow fibers. Most dialyzers used in adults have a surface area between 1.5 and 2.1 m2. Parallel plate dialyzers are rarely used today. Most dialysis membranes in use today are made from a variety of synthetic materials including polyamide, polymethylmethacrylate, acrylnitrile-sodium methallylsulfonate (AN-69), polyacrylonitrile, polycarbonate, and polysulfone. Cellulose membranes are being used with decreasing frequency in the United States.
The contact of blood with the membrane can result in activation of the complement system, with the release of bradykinin or cytokines. The biocompatibility of the dialysis membrane depends not only on the material used but also on the degree of blood contact with the dialysate. Unsubstituted cellulose membranes activate the complement system. To decrease complement activation, the hydroxyl groups of cellulose have been replaced with acetate or a synthetic material has been added to cellulose.
A high efficiency membrane has the ability to remove small solutes well. The removal of small solutes is a function of the membrane surface area. High-efficiency membranes have a large surface area. The efficiency of a dialyzer is measured by the clearance of urea (MW 60) and is expressed as KoAurea. Larger molecular-weight solutes are removed to a greater degree by membranes with larger membrane pores. These membranes are referred to as high-flux membranes. High-flux and many high-efficiency membranes also have the ability to achieve a high ultrafiltration rate. The water permeability of a membrane is specified by its ultrafiltration coefficient (Kuf).
The clearance of creatinine (MW 113) by a dialyzer is usually about 20% less than the dialyzer urea clearance, despite the minimal difference in molecular weight. The removal of phosphorus (MW 31) by dialysis depends mostly on the time provided for dialysis per week, and also on the dialyzer efficiency and the predialysis phosphorus level. During dialysis, phosphorus is removed rapidly from plasma but not from the intracellular compartment. The slow equilibration between these compartments and bone is the major limiting factor of phosphorus removal.
Historically, middle molecule clearance was defined by the clearance of vitamin B12 (MW 1355). However, the clearance of vitamin B12 is low due to its high degree of protein binding. Thus, many high-flux dialyzers are now classified based on the clearance of molecules such as β2-microglobulin (MW 11,800). With the introduction of high-flux dialyzers, the clearance of β2-microglobulin has improved. Despite these improvements, the serum concentration of β2-microglobulin remains markedly elevated in anuric hemodialysis patients using high-flux dialyzers. β2-Microglobulin deposition is the cause of dialysis-associated amyloidosis.
Reuse of dialyzers is a common practice in outpatient dialysis units in the United States, but is less common in other countries. A method for the reuse process used in the United States has been written by the Association for the Advancement of Medical Instrumentation (AAMI). Each dialyzer should be labeled with the patient’s identifying information. The total cell volume (TCV) should be measured prior to its first use. After the dialysis treatment, the membrane is rinsed with normal saline, pressure washed, and then cleansed with either bleach or a hydrogen peroxide mixture. Bleach can damage the membrane and increase protein loss with dialysis if it is used in inappropriately high concentrations. Once the membrane has been cleaned, its performance is evaluated by measuring the TCV. If the new value for TCV is >80% of the original TCV, it passes the performance test, and the membrane can be reused after disinfection and sterilization with a mixture of hydrogen peroxide, formaldehyde, or glutaraldehyde. The polysulfone membranes can also be heat sterilized. The final step of the reuse process is the removal of the germicide. Residual germicide can cause a burning sensation, itching, or other allergic reactions. The reuse of dialyzers needs an informed consent. Patients with bacteremia or hepatitis B are excluded from dialyzer reuse. HIV and hepatitis C infection are not considered contraindications to reuse. In general, membrane biocompatibility improves with dialyzer reuse. Exposure of the membrane to blood can result in the protein coating of the membrane. This protein coating may decrease complement activation. Dialyzers can be reused dozens of times without a significant loss of efficacy. A decrease in the reuse number may suggest an increased rate of clotting of the hollow fibers and can often be improved by adjusting the anticoagulation prescription.
The blood pump moves the blood from the arterial line through the dialyzer back to the venous line. The speed of the blood pump can be adjusted to between 200 and 600 mL/minute. At any given time about 200–250 mL of blood is outside the patient. The dialysis pump sucks the dialysis fluid (dialysate) away from the dialyzer producing the transmembrane pressure. The transmembrane pressure can be adjusted to achieve the desired fluid removal. In modern dialysis machines, the transmembrane pressure is automatically adjusted by the dialysis machine based upon the amount of volume to be removed during the dialysis session and the type of ultrafiltration profiling chosen. The dialysate flow rate is usually between 500 and 800 mL/minute and is usually set between 100 and 200 mL/minute higher than the blood flow rate. The dialysate temperature can be adjusted. A lower temperature can cause peripheral vasoconstriction in the patient and thus improve hemodynamic stability.
The arterial and venous pressures are monitored during the dialysis treatment. The arterial pressure is measured before the blood pump to avoid excessive suction of blood and the venous pressure is measured before the blood returns to the access to avoid excessive resistance. A high venous pressure in the access is suggestive of an impairment to flow in the venous outflow tract that could be due to stenosis in the venous outflow of the access, clotting in the venous chamber of the catheter, stenosis in native vessels through which the access drains, or kinking of the blood lines. A high negative arterial pressure is indicative of immature access, stenosis or scarring in the accessed area, suctioning against the vessel wall, or the use of long or small gauge needles or catheters.
Other safety guards included on dialysis machines are the air trap to detect air embolism, the blood leak detector to detect blood in the dialysate compartment, and the measurement of dialysate conductivity to detect a malfunction in mixing the dialysis solution. If one of these safety guards is triggered, the machine will alarm and in some cases shut down. If a blood leak is detected, the dialyzer will be replaced and the patient will be administered antibiotics to treat any possible contamination of blood with the dialysis solution.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree