Hypercalcemia
- Hypercalcemia is usually manifested as a chronic but mildly elevated serum calcium level, although more severe forms that present as hypercalcemic emergencies do exist.
- The symptoms associated with sustained hypercalcemia are relatively nonspecific, but the constellation of symptoms often suggests the diagnosis.
- A combination of neuropsychiatric complaints such as depression, anxiety, cognitive dysfunction, headache, fatigue and even organic brain syndrome, renal complaints including polyuria, polydipsia, nephrogenic diabetes insipidus, nephrolithiasis, nocturia, and renal insufficiency, and gastrointestinal complaints such as constipation, peptic ulcer disease, or a diagnosis of acute pancreatitis would strongly suggest the diagnosis.
- Most patients with hypercalcemia are diagnosed based on data derived from laboratory screening tests.
- The signs and symptoms associated with the underlying disease causing hypercalcemia may dominate the clinical picture.
Calcium in serum exists ionized, bound to organic anions such as phosphate and citrate, and bound to proteins (mainly albumin). Of these, ionized calcium is the physiologically important form. The most common abnormality that distorts the relationship between serum calcium and ionized calcium is hypoalbuminemia. The total serum calcium is lower or higher by 0.8 mg/dL (0.2 mmol/L) for every 1.0 g/dL that the serum albumin is higher or lower, respectively, than 4 g/dL. Thus, patients may have a normal serum ionized calcium but low total calcium if they have hypoalbuminemia due to nephrotic syndrome. Conversely, a patient can have high total calcium, with normal ionized calcium and increased total protein and/or albumin, as in states of severe dehydration.
Hypercalcemia is one of the most common metabolic disorders in malignant diseases and develops in 3–30% of such patients. Hypercalcemia of malignancy is the most common cause of hypercalcemia followed by primary hyperparathyroidism in hospital populations. The most common cause in normal populations is primary hyperparathyroidism followed by transient hypercalcemia.
Hypercalcemia can result from increased bone resorption, decreased renal excretion, or increased gastrointestinal absorption. However, bone resorption and intestinal hyperabsorption of calcium are the predominant causes of hypercalcemia. Reduced renal excretion is a permissive factor in all cases of hypercalcemia as in the absence of renal conservation of calcium, any rise in serum calcium would result in the excretion of any excess in the urine and hypercalciuria but not hypercalcemia would ensue.
Typically, the mechanism underlying hypercalcemia is complex and multifactorial. In primary hyperparathyroidism, all three components come into play. High parathyroid hormone (PTH) levels induce bone resorption, increase renal tubular reabsorption, and secondarily increase gastrointestinal calcium absorption as PTH stimulates production of the most active form of vitamin D, calcitriol.
PTH is the master hormone regulating overall calcium metabolism. It is an 84-amino acid hormone that in response to a fall in serum calcium levels raises calcium levels by accelerating osteoclastic bone resorption and increasing renal tubular resorption of calcium. It also increases calcitriol, which indirectly raises serum calcium levels. PTH also induces an increased renal excretion of phosphate. This effect helps to enhance the rise in serum calcium as phosphate tends to coprecipitate with calcium and block the effects of PTH on bone and the effects of PTH on inducing the activation of vitamin D by the kidney.
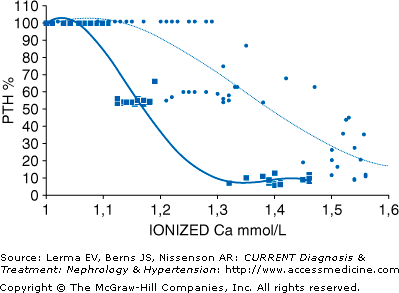
In primary hyperparathyroidism, there is a fundamental dysregulation of PTH secretion. Normally, the calcium-sensing receptor on the surface of cells in the parathyroid gland senses serum calcium and the release of PTH follows a sigmoidal relationship concentration (see Figure 6–1). The “set point” of this relationship is the serum calcium concentration at which there is half-maximal inhibition of PTH secretion. As seen in Figure 6–1, this relationship is disrupted in primary hyperparathyroidism so that PTH is released even in the face of normally suppressive levels of serum calcium.
Figure 6–1.
The set point of calcium (calculated as the midpoint between maximal and minimal PTH secretion), the serum ionized calcium level at maximal PTH secretion, and the serum ionized calcium at maximal PTH inhibition are shown for individual patients affected by primary hyperparathyroidism (n = 19, circles) and for 14 normal subjects (squares). The sigmoidal curve is shifted to the right in primary PTH. (Reprinted with permission from Malberti F et al: The PTH-calcium curve and the set point of calcium in primary and secondary hyperparathyroidism. Nephrol Dial Transplant 1999;14:2398.)
Vitamin D is a steroid hormone that may be ingested with the diet but is also produced in the skin by the action of sunlight on metabolic antecedents of vitamin D. Calcitriol, the active form of vitamin D, is derived from the hydroxylation of cholecalciferol, which is first hydroxylated in the liver to 25-hydroxyvitamin D, then in the kidneys to 1,25-dihydroxyvitamin D. Vitamin D has a plethora of actions including altering the growth dynamics of many cell types. Its actions to increase serum calcium are complex and include an increase in the transport of calcium across the gastrointestinal (GI) tract and an increase in calcium release from bone during PTH-induced bone resorption. In the absence of PTH, the GI effect in concert with adequate dietary calcium can maintain normal serum calcium levels and with pharmacologic doses of vitamin D, even induce hypercalcemia.
PTH-related peptide (PTHrP) is the principal mediator in hypercalcemia associated with solid tumors. Patients with humoral hypercalcemia of malignancy (HHM) constitute about 80% of all patients with hypercalcemia associated with malignancy. PTHrP and PTH share the same molecular region that comprises the receptor-binding domain at the amino terminus. PTHrP binds the PTH receptor and mimics the biologic effects of PTH on bones and the kidneys. PTHrP and PTH share the same receptor, but there are some differences in actions. HHM patients have a greater degree of hypercalciuria than is generally seen in hyperparathyroidism. HHM is usually associated with low serum calcitriol levels, whereas PTH stimulates calcitriol production. Also, PTH stimulates bone resorption and formation, whereas PTHrP stimulates only bone resorption, with very low osteoblastic activity, and thus usually normal alkaline phosphatase levels.
Bone resorption is a key mechanism underlying most cases of hypercalcemia. The skeleton is continually renewed through remodeling, a sequence of events whereby old bone is replaced by new bone (bone turnover). Three types of cells produce and maintain bone. Osteoblasts act at bone surfaces by secreting osteoid, unmineralized collagen, and modulate the crystallization of hydroxyapatite and influence the activity of osteoclasts. Osteoclasts are responsible for the resorption of bone, a process that is necessary for the repair of bone surfaces and the remodeling of bone. Osteocytes are osteoblasts that have become embedded within the mineralized regions of bone.
During bone growth, formation is higher than breakdown. After peak bone mass is achieved, the rates of breakdown and formation are equal and bone mass is thought to remain constant. In hypercalcemic states associated with excess PTH, PTHrP, and other bone-active cytokines, the rate of breakdown increases and exceeds the rate at which bone is formed. If this is coupled with inadequate renal excretion, then hypercalcemia ensues.
Primary hyperparathyroidism, the most common cause of hypercalcemia in the outpatient setting, is usually found by routine laboratory screening, as most patients with primary hyperparathyroidism are asymptomatic. When patients are symptomatic, findings may include renal calculi, bone pain, pathologic fractures, and proximal muscle weakness, or nonspecific symptoms such as depression, lethargy, and vague aches and pains. Rarely, full blown psychiatric disorders may be seen. Occasional patients may have a family history of multiple endocrine neoplasia syndromes (type 1 or 2) or a history of preceding head and neck irradiation as a child or an adult for hyperthyroidism treated with radiation modalities. Mental obtundation or coma is an infrequent but life-threatening complication of severe hypercalcemia (hypercalcemic crisis). All patients with calcium-containing renal stones should be evaluated for primary hyperparathyroidism.
Primary hyperparathyroidism is usually diagnosed by demonstrating persistent hypercalcemia in the presence of inappropriately normal or elevated PTH concentrations. Normally, PTH levels are suppressed in the presence of increasing serum calcium levels. Immunoassay of the intact PTH molecule is the preferred method of measurement. Primary hyperparathyroidism may present at an early stage with minimal increases in the serum calcium level in the face of normal or mildly elevated PTH levels. The intact PTH assay uses antibodies to two sites simultaneously (N- and C-terminal) to measure the intact hormone. The normal range for this assay is 18–65 pg/mL. Intact PTH is increased in 80–90% of patients with primary hyperparathyroidism while 10–20% have values in the normal range but that are inappropriately high in the face of hypercalcemia. The intact PTH assay provides good discrimination between parathyroid and nonparathyroid causes of hypercalcemia. This diagnosis should be suspected in patients with renal calculi or reduced bone density who have minimally increased PTH levels and high-normal serum calcium levels (normocalcemic primary hyperparathyroidism). Other findings include mild hypophosphatemia, hypercalciuria, and mild metabolic acidosis as PTH acts on the kidney to promote phosphate and bicarbonate excretion.
If a parathyroid adenoma is suspected, the parathyroid glands can be imaged using nuclear medicine techniques. This procedure is based on the differential uptake between normal and abnormal parathyroid glands of the tumor-seeking compound sestamibi, labeled with 99mTc. This material is taken up by both the thyroid and parathyroids and is cleared rapidly from normal tissue but is retained in tumors of the parathyroids. Surgical exploration and the removal of the adenomatous gland constitute the treatment of choice for many patients. Preoperative imaging may not always be necessary since the success rate for parathyroid surgery has been reported to exceed 90%, even in the absence of prior imaging. Localization techniques are more useful in cases of failed primary neck exploration and in cases of postoperative persistent hypercalcemia.
Familial hypocalciuric hypercalcemia is characterized by benign asymptomatic hypercalcemia. It is an autosomal dominant condition in which the genetic abnormality leads to a loss of function mutation of the calcium-sensing receptor that exists on the c-cells of the parathyroid glands. This inactive receptor necessitates higher serum calcium levels in order to suppress PTH. Because the calcium-sensing receptor also exists within the kidney and regulates calcium reabsorption in the thick ascending loop of Henle, the defect in the protein structure leads to increased renal tubular calcium reabsorption and reduced excretion. Apparently many of the symptoms associated with hypercalcemia require activation of the calcium-sensing receptor as patients with this condition are remarkably free of symptoms. It is therefore important to inquire about familial hypercalcemia in any hypercalcemic patients and to assess urinary calcium excretion.
Strikingly low urinary calcium excretion (<100 mg/24 hours) is seen in the majority of patients, despite hypercalcemia and iPTH levels in the normal range in 80–85% of patients.
Hypercalcemia of malignancy occurs in 10–20% of patients with cancer and the hypercalcemia is usually severe and of relatively short duration. The tumors that manifest in this disorder most commonly are breast, lung cancer, and multiple myeloma. The condition is induced by a number of humoral mediators: PTHrP is the most common and is found in 80–90% of cases and in both carcinomas and lymphomas. In multiple myeloma, the mediator is osteoclast-activating factor. In those patients with Hodgkin’s disease and a substantial fraction of those with non-Hodgkin’s lymphomas who develop hypercalcemia, high levels of calcitriol are found. The malignant cells are capable of enzymatically activating 25-hydroxyvitamin D to its more active metabolite calcitriol. In patients with osteolytic metastases, a variety of cytokines released by the tumor cells including tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, and PTHrP may each lead to activation of bone resorption and, ultimately, hypercalcemia.
Generally, patients with HHM manifest higher levels of serum calcium than those with primary hyperparathyroidism. Also, serum phosphate and bicarbonate levels tend to be higher. However, specific laboratory tests are rarely required for the diagnosis of HHM since patients with malignancy almost always have other signs or symptoms of their malignancy when hypercalcemia is found. However, assays for iPTH and for PTHrP are available and finding an elevated PTHrP is specific for HHM.
Hypercalcemia occurs in most granulomatous disorders. High serum calcium levels are seen in about 10% of patients with sarcoidosis; hypercalciuria is about three times more frequent. Tuberculosis, fungal diseases including histoplasmosis, cocidioidomycosis, and berylliosis, and some lymphomas, particularly those associated with human immunodeficiency viruses, are other conditions that are associated with disorders of calcium metabolism. These abnormalities of calcium metabolism are due to production of calcitriol by activated macrophages either in pulmonary alveoli or in granulomatous inflammation. Macrophages have the enzymatic capacity to convert 25-hydroxyvitamin D3 to calcitriol. As this condition is associated with suppressed PTH levels, hypercalciuria is a prominent feature of this form of hypercalcemia.
The milk-alkali syndrome became rare with the advent of modern gastric ulcer therapy with antibiotics. However, the growing popularity of the use of calcium carbonate as an antacid or as calcium supplementation to prevent osteoporosis has led to a reappearance of this problem. Patients with this syndrome typically ingest massive quantities of calcium and absorbable alkali and are unaware of the toxic effects of these compounds. They present with the triad of hypercalcemia, metabolic alkalosis, and renal failure that is occasionally so severe that dialysis is necessary. The serum parathyroid hormone or calcitriol levels are appropriately decreased in response to the hypercalcemia. Since both physicians and patients are often unaware of the calcium and alkali content of many nonprescription medicines, the diagnosis of the milk-alkali syndrome, a reversible cause of renal failure, can be missed if a detailed history of such intake is not elicited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree