T Cell Activation and the Immunologic Synapse: Signal One
The immunologic synapse consists of a multiplicity of T cell-surface protein forms and clusters, thereby creating a platform for antigen recognition and generation of crucial T cell activation-related signals.
2 The synapse begins to form when the initial adhesions between certain T cell (e.g., CD2, LFA-1) and APC surface proteins (e.g., CD58, ICAM-1) are formed (
Table 81.2). These physical contacts between T cells and APCs provide an opportunity for the antigen reactive T cells to recognize cognate antigen. Antigen-driven T cell activation, a highly coordinated, preprogrammed process, begins when T cells recognize intracellularly processed fragments of foreign proteins (approximately 8 to 16 amino acids) embedded within the groove of the major histocompatibility complex (MHC) proteins expressed on the surface of APCs.
3,
4,
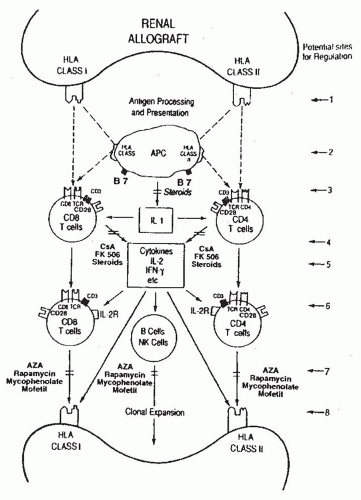
5 Some of the recipient’s T cells directly recognize the allograft (i.e., donor antigen [s] presented on the surface of donor APCs) and this process is termed direct recognition whereas other T cells recognize the donor antigen after it is processed and presented by self-APCs
6 (
Fig. 81.1) and this process is designated indirect recognition.
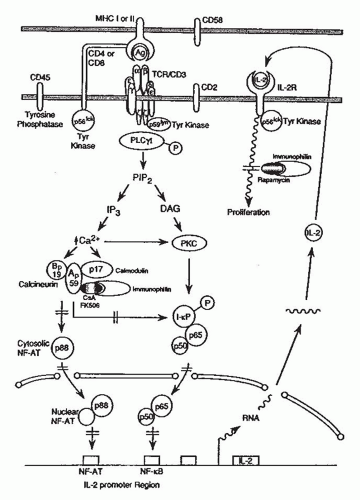
The T cell antigen receptor (TCR)-CD3 complex is composed of clonally distinct TCR α and β peptide chains that recognize the antigenic peptide in the context of MHC proteins and clonally invariant CD3 chains that propagate intracellular signals originating from antigenic recognition (
Fig. 81.2).
2,
7,
8 The TCR variable, diversity, junction, and constant region genes (i.e., genes for regions of the clonespecific antigen receptors) are spliced together in a cassettelike fashion during T cell maturation.
7 A small population of T cells expresses TCR γ and σ chains instead of the TCR α and β chains.
CD4 and CD8 proteins, expressed on reciprocal T cell subsets, bind to nonpolymorphic domains of human leukocyte antigen (HLA) class II (DR, DP, DQ) and class I (A, B, C) molecules, respectively (
Fig. 81.1 and
Table 81.2).
2,
7 A threshold of TCR to MHC-peptide engagements is necessary to stabilize the immunologic synapse stimulating a redistribution of cell-surface proteins and coclustering of the TCR/CD3 complex with the T cell-surface proteins.
8,
9,
10 Additional T cell surface proteins such as CD5 proteins join the synapse.
9,
10 The TCR cluster already includes integrins (e.g., LFA-1) and nonintegrins (e.g., CD2)
2,
8,
9 that have created T cell-APC adhesions. Hence, antigen recognition stimulates a redistribution of cell-surface proteins and coclustering of the TCR/CD3 complex with the T cell-surface proteins
2,
7,
8,
9 and signaling molecules. This multimeric complex functions as a unit in initiating T cell activation.
Following activation by antigen, the TCR-CD3 complex and coclustered CD4 and CD8 proteins are physically associated with intracellular protein-tyrosine kinases (PTKs)
of two different families, the src (including p59
fyn and p56
lck) and ZAP 70 families.
2 The CD45 protein, a tyrosine phosphatase, contributes to the activation process by dephosphorylating an autoinhibitory site on the p56
lck PTK. Intracellular domains of several TCR/CD3 proteins contain activation motifs that are crucial for antigen-stimulated signaling. Certain tyrosine residues within these motifs serve as targets for the catalytic activity of src family PTKs. Subsequently, these phosphorylated tyrosines serve as docking stations for the SH2 domains (recognition structures for select phosphotyrosinecontaining motifs) of the ZAP-70 PTK. Following antigenic engagement of the TCR/CD3 complex, select serine residues of the TCR and CD3 chains are also phosphorylated.
2,
5The waves of tyrosine phosphorylation triggered by antigen recognition encompass other intracellular proteins and are a cardinal event in initiating T cell activation. Tyrosine phosphorylation of the phospholipase Oγ1 activates this coenzyme and triggers a cascade of events that leads to full expression of T cell programs: hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) and generation of two intracellular messengers, inositol 1,4,5-triphosphate (IP
3) and diacylglycerol (
Fig. 81.2).
11 IP
3, in turn, mobilizes ionized calcium from intracellular stores, while diacylglycerol, in the presence of increased cytosolic free Ca
2+, binds to and translocates protein kinase C (PKC)—a phospholipid/Ca
2+-sensitive protein serine/threonine kinase—to the membrane in its enzymatically active form.
5,
11 Sustained activation of PKC is dependent on diacylglycerol generation from hydrolysis of additional lipids, such as phosphatidylcholine.
The increase in intracellular free Ca
2+ and sustained PKC activation promote the expression of several nuclear regulatory proteins (e.g., nuclear factor of activated T cells
[NF-AT], nuclear factor kappa B [FN-κB] , activator protein 1 [AP-1]) and the transcriptional activation and expression of genes central to T cell growth (e.g., interleukin-2 [IL-2] and receptors for IL-2 and IL-15).
2,
5,
12Calcineurin, a Ca
2+– and calmodulin-dependent serine/threonine phosphatase, is crucial to Ca
2+-dependent, TCR-initiated signal transduction.
13,
14 Inhibition by cyclosporine and tacrolimus (FK-506) of the phosphatase activity of calcineurin is considered central to their immunosuppressive activity
15
Costimulatory Signals: Signal Two
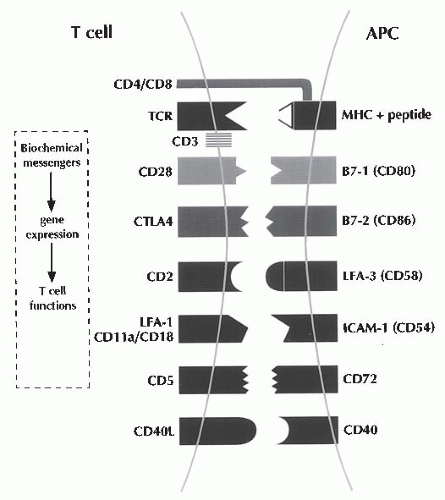
Signaling of T cells via the TCR/CD3 complex (signal one) is necessary, but insufficient, to induce T cell proliferation; full activation of T cells is dependent on both the antigenic signals and the costimulatory signals (signal two) engendered by the contactual interactions between cell-surface proteins expressed on antigen-specific T cells and APCs (
Fig. 81.3 and
Table 81.2).
16,
17 The interaction of the CD2 protein on the T cell surface with the CD58 (leukocyte function-associated antigen 3 [LFA-3]) protein on the surface of APCs, and that of the CDlla/CD18 (LFA-1) proteins with the CD54 (intercellular adhesion molecule 1 [ICAM-1]) proteins,
18 and/or the interaction of the CD5 with the CD72 proteins
10 aids in imparting such a costimulatory signal.
Recognition of the B7-1 (CD80) and B7-2 (CD86) proteins expressed upon CD4+ T cells generates a very
powerful T cell costimulus.
19 A subset of monocytes and dendritic cells constitutively express CD80 and CD86 at low levels and cytokines (e.g., granulocyte-macrophage colony-stimulating factor [GMCSF] or interferon-γ [IFN-γ]) stimulate heightened expression of CD80 and CD86 on monocytes, B cells, and dendritic cells.
19 Many T cells express B7-binding proteins (i.e., CD28 proteins that are constitutively expressed on the surface of CD4 + T cells and CTLA-4 [CD152]), a protein whose ectodomain is closely related to that of CD28, and is expressed upon activated CD44- and CD84- T cells. CD28 binding of B7 molecules stimulates a Ca
2+-independent activation pathway that leads to stable transcription of the IL-2, IL-2 receptors, and other activation genes resulting in vigorous T cell proliferation.
19 For some time, the terms CD28 and the costimulatory receptor were considered synonymous by some, but the demonstration that robust T cell activation occurs in CD28-deficient mice indicated that other receptor ligand systems contribute to signal two.
20 In particular, the interaction between CD40 expressed upon APCs and CD40 ligand (CD154) expressed by antigen-activated CD44- T cells has received great attention as a potent second signal.
21The delivery of the antigenic first signal and the costimulatory second signal leads to stable transcription of the IL-2, several T cell growth-factor receptors, and other pivotal T cell activation genes (
Table 81.2). The Ca
2+-independent costimulatory CD28 pathway is relatively more resistant to inhibition by cyclosporine or FK-506 as compared to the calcium-dependent pathway of T cell activation. Whereas the interactions between B7 proteins and its counter receptor CD28 result in positive costimulation, the interactions between B7 proteins by CTLA-4, a protein primarily expressed on activated T cells, result in the generation of a negative signal to T cells. This coinhibitory signal is a prerequisite for peripheral T cell tolerance.
22The formulation that full T cell activation is dependent on the costimulatory signal, as well as the antigenic signal, is most significant, as T cell molecules responsible for costimulation and their cognate receptors on the surface
of APCs then represent target molecules for the regulation of the antiallograft response. Indeed, transplantation tolerance has been induced in experimental models by targeting a variety of cell-surface molecules that contribute to the generation of costimulatory signals, and tolerance to histoincompatible human kidney allografts has been accomplished with a conditioning regimen that includes monoclonal antibodies directed at the CD2 protein.
23
lnterleukin-2/lnterleukin-l 5 Stimulated TCell Proliferation
Autocrine type of T cell proliferation occurs as a consequence of the T cell activation-dependent production of IL-2 and the expression of multimeric high affinity IL-2 receptors on T cells (
Fig. 81.2) formed by the noncovalent association of three IL-2-binding peptides (α β, γ).
12,
24,
25,
26 IL-15 is a paracrine-type T cell-growth factor family member with very similar overall structural and identical T cell stimulatory qualities to IL-2.
12 The IL-2 and IL-15 receptor complexes share β and γ chains that are expressed in low abundance upon resting T cells; expression of these genes is amplified in activated T cells. The α-chain receptor components of the IL-2 and IL-15 receptor complexes are distinct and expressed upon activated, but not resting, T cells. The intracytoplasmic domains of the IL-2 receptor β and γ chains are required for intracellular signal transduction. The ligand-activated, but not resting, IL-2/IL-15 receptors are associated with intracellular PTKs.
12,
27,
29 Raf-1, a protein serine/threonine kinase associates with the intracellular domain of the shared β chain,
30 and this association and the kinase activity are prerequisites to IL-2/IL-15-triggered cell proliferation. Translocation of IL-2 receptor-bound Raf-1 serine/threonine kinase into the cytosol requires IL-2/IL-15-stimulated
PTK activity. The ligand-activated common γ chain recruits a member of the Janus kinase family, Jak 3, to the receptor complex that leads to activation of a member of the STAT family Activation of this particular Jak-STAT pathway is essential for the proliferation of antigen-activated T cells. The subsequent events leading to IL-2/IL-15-dependent proliferation are not fully resolved; however, IL-2/IL-15—stimulated expression of several DNA binding proteins including Bcl-2, c-jun, c-fos, and c-myc contributes to cell cycle progression.
31,
32 It is interesting and probably significant that IL-2, but not IL-15, triggers apoptosis of many antigen-activation T cells. In this way, IL-15-triggered events may be more detrimental to the antiallograft response than those initiated by IL-2. As IL-15 is not produced by T cells, IL-15 expression is not regulated by cyclosporine or tacrolimus.
Humoral Rejection
Antibody-mediated rejection (AMR) is a form of humoral rejection wherein antibodies directed at the donor HLA antigens (DSAs) serve as the main effector for the immune response directed at the allograft. Antibodies directed at non-HLA antigens such as endothelial cell associated antigens and MHC class I-related chain A antigens (MICA) have also been implicated in the pathogenesis of AMR. Whereas most acute T cell mediated rejections (TMRs) are responsive to steroid therapy, AMR is typically steroid-resistant and requires additional treatment such as plasmapheresis, anti-B cell, and intravenous immunoglobulin (IVIG) therapy. The incidence of AMR has been estimated at less than 10% but appears to be on the rise due to multiple reasons including acute TMR being effectively prevented by current immunosuppressive regimens, better definition of AMR, and transplantation of individuals with humoral presenitization and repeat transplants. Patients with AMR invariably harbor anti-HLA DSA although, in certain cases, histopathologic evidence of AMR may be apparent without any anti-HLA DSA. Acute AMR may occur within 1 week after engraftment even in the setting of antithymocyte globulin induction therapy. The diagnosis of AMR requires the presence of C4d complement staining in the peritubular capillaries in addition to peritubular capillary inflammation with polymorphonuclear and mononuclear leukocytes or the presence of fibrinoid changes/transumural arterial inflammation or acute tubular necrosis (ATN)-like tissue injury.
33 In the current Banff classification schema, those who present with histolgic features consistent with AMR but without concurrent intragraft C4d deposition or circulating DSA are classified as supicious for AMR—it is possible that the offending antibodies may be of the noncomplement fixing IgG subtypes and/or non-HLA antibodies (because most screening assays for DSA utilize HLA as target antigens).
A novel form of humoral rejection has also been documented. Antibodies directed against two epitopes of the angiotensin II type I (ATO receptor have been associated with refractory vascular allograft rejection in a series of 16 patients and these patients did not have anti-HLA antibodies at the time of incident humoral rejection.
34
Immunobiology and Molecular Diagnosis of Rejection
The net consequence of cytokine production and acquisition of cell-surface receptors for these transcellular molecules is the emergence of antigen-specific and graft-destructive T cells
and antibody producing B cells/plasma cells (
Fig. 81.1). Cytokines facilitate not only the T cell effector arm and TCR but also the B cell/plasma cell arm by promoting the production of cytopathic antibodies. Moreover, cytokines such as IFN-y and tumor necrosis factor-α (TNF-α) can amplify the ongoing immune response by upregulating the expression of HLA molecules as well as costimulatory molecules (e.g., B7) on graft parenchymal cells and APCs (
Fig. 81.1). We and others have demonstrated the presence of antigen-specific cytotoxic T lymphocytes (CTL) and anti-HLA antibodies during or preceding a clinical rejection episode.
35,
36 We have detected messenger RNA (mRNA) encoding the CTL-selective serine protease (granzyme B), perforin, Fasligand attack molecules, and immunoregulatory cytokines, such as IL-10 and IL-15, in human renal allografts undergoing acute rejection.
37 Indeed, these gene expression events may anticipate clinically apparent rejection. More recent efforts to develop a noninvasive method for the molecular diagnosis of rejection have proved rewarding. Using either peripheral blood
38 or urinary leukocytes
39 rejection-related, gene expression events evident in renal biopsy specimens are robustly detected in peripheral blood or urinary sediment specimens. Initial results from large-scale multicenter trials (e.g., Clinical Trials in Organ Transplantation, CTOT-04) support the hypothesis that noninvasive diagnosis of acute TMR is feasible by measurement of genes encoding cytotoxic attack molecules in urine, and the urinary cell mRNA profiles may anticipate the future development of acute TMR.
40 We speculate as well that a noninvasive, molecular diagnostic approach to rejection would be of value toward the detection of insidious, clinically silent rejection episodes that, although rarely detected through standard measures, are steroid-sensitive but usually lead to chronic rejection.
41