Chapter 9 PELVIC FLOOR ULTRASOUND
The increasing availability of ultrasound and magnetic resonance imaging (MRI) equipment has triggered a renewed interest in diagnostic imaging in female urology and urogynecology, after radiologic methods, developed since the 1920s,1–6 had largely fallen into disuse. Because of cost and access problems, MRI has had limited clinical use in the evaluation of pelvic floor disorders, and until recently, slow acquisition speeds have precluded dynamic imaging. In contrast, ultrasound is almost universally available and provides real-time observation of diagnostic maneuvers. Beginning in the 1980s, transabdominal,7,8 perineal,9,10 transrectal,11 and transvaginal ultrasound12 have been investigated for use in women with urinary incontinence and pelvic organ prolapse. Because of its noninvasive nature, ready availability, and the absence of distortion, perineal or translabial ultrasound has become the most widely used method.
The development of three-dimensional (3D) ultrasound13,14 has opened up new diagnostic possibilities. The first attempts at producing 3D-capable systems were made in the 1970s, when processing a single volume of data required 24 hours of computer time on a system that filled a small room.15 Such data processing is now possible on a laptop computer and is achieved in real time. Transvaginal, transrectal, and translabial 3D ultrasound techniques have been reported, and significant development in this field is likely to occur in the next few years.
TWO-DIMENSIONAL PELVIC FLOOR ULTRASOUND
Basic Methodology
A midsagittal view is obtained by placing a transducer (usually a curved array with frequencies between 3.5 and 9 MHz) on the perineum (Fig. 9-1) after covering the transducer with a glove or thin plastic wrap for hygienic reasons. Powdered gloves can markedly impair imaging quality because of reverberations and should be avoided. Imaging can be performed with the patient in the dorsal lithotomy position, with the hips flexed and slightly abducted, or in the standing position. Bladder filling should be specified; for some applications, prior voiding is preferable, and a full bladder can prevent complete development of a prolapse. The presence of a full rectum may also impair diagnostic accuracy and sometimes necessitates a repeat assessment after bowel emptying. Parting of the labia can improve image quality, which is optimal in pregnancy and poorest in menopausal women with marked atrophy, most likely due to various levels of tissue hydration. The transducer usually can be placed quite firmly against the symphysis pubis without causing significant discomfort, unless there is marked atrophy. The resulting image includes the symphysis pubis (specifically, the interpubic disk) anteriorly, the urethra and bladder neck, the vagina, cervix, rectum, and anal canal (see Fig. 9-1) with the internal and external anal sphincter. Posterior to the anorectal junction, a hyperechogenic area indicates the central portion of the levator plate, the puborectalis-pubococcygeus (or pubovisceral) muscle. The cul-de-sac may also be seen as filled with a small amount of fluid, echogenic fat, or peristalsing small bowel. Parasagittal or transverse views may yield additional information, such as enabling assessment of the puborectalis muscle and its insertion on the on the pelvic sidewall and imaging of transobturator implants.
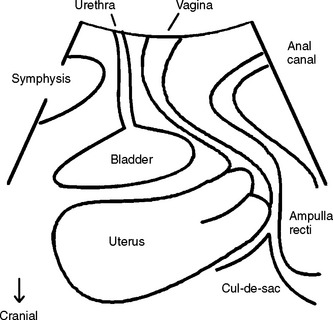
Figure 9-1 Drawing of the field of vision for translabial or perineal ultrasound in the midsagittal plane.
Image adapted from Ultrasound Obstet Gynecol 2004; 23:80-92, with permission.
There has been disagreement regarding image orientation in the midsagittal plane. Some clinicians prefer orientation as in the standing patient facing right,16 which requires image inversion on the ultrasound system, a facility that is not universally available. Others (including me) prefer an orientation as on conventional transvaginal ultrasound (i.e., cranioventral aspects to the left, dorsocaudal to the right). The latter orientation seems more convenient when using 3D or 4D systems because it automatically results in rendered volumes that are oriented as on conventional MRI of the pelvic floor (discussed later). Because any image reproduced in one of these orientations can be converted to the other by rotation through 180 degrees, formal standardization may be unnecessary. Orientations that require mirroring for conversion should be avoided.
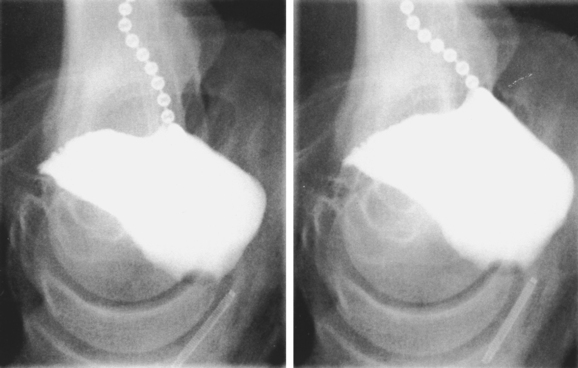
Translabial ultrasound of the lower urinary tract, even if limited to B-mode imaging in the midsagittal plane, yields information equivalent or superior to the lateral urethrocystogram (Fig. 9-2, shown rotated by 180 degrees for comparison) or fluoroscopic imaging. Comparative studies have mostly shown good correlation between radiologic and ultrasound data.11,17–22 The one remaining advantage of x-ray fluoroscopy may be the ease with which the voiding phase can be observed, although some investigators have used specially constructed equipment to document voiding with ultrasound.23
Bladder Neck Position and Mobility
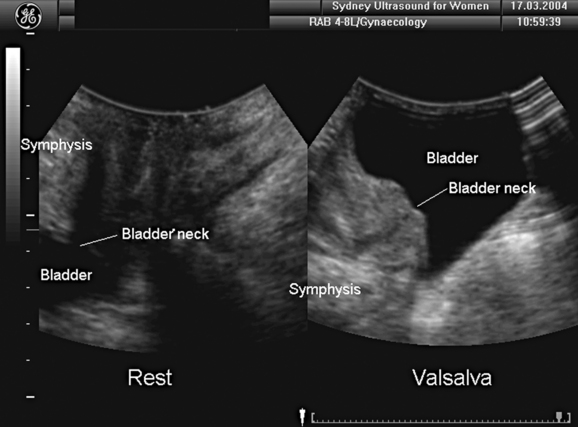
Bladder neck position and mobility can be assessed with a high degree of reliability. Points of reference are the central axis of the symphysis pubis24 or its inferoposterior margin.17 The former may be more accurate because measurements are independent of transducer position or movement; however, because of calcification of the interpubic disk, the central axis is often difficult to obtain in older women, reducing reliability. Imaging can be undertaken with the patient supine or erect and with a full or empty bladder. The full bladder is less mobile25 and may prevent complete development of pelvic organ prolapse. In the standing position, the bladder is situated lower at rest but descends about as far as in the supine patient during a Valsalva maneuver.26 Either way, it is essential to not exert undue pressure on the perineum to allow full development of pelvic organ descent, although this may be difficult in women with severe prolapse, such as vaginal eversion or procidentia.
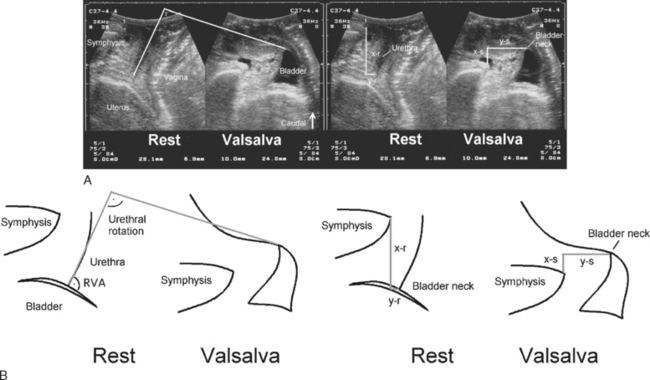
Measurements of bladder neck position are generally performed at rest and during maximal Valsalva maneuver. The difference yields a numeric value for bladder neck descent. During a Valsalva maneuver, the proximal urethra may rotate in a posteroinferior direction. The extent of rotation can be measured by comparing the angle of inclination between the proximal urethra and any other fixed axis (Fig. 9-3). Some investigators measure the retrovesical (or posterior urethrovesical) angle between proximal urethra and trigone (see Fig. 9-3).27 Others determine the angle γ between the central axis of the symphysis pubis and a line from the inferior symphyseal margin to the bladder neck.28,29 Of all the ultrasound parameters of hypermobility, bladder neck descent may have the strongest association with urodynamic stress incontinence.30 The reproducibility of this dynamic measurement has been assessed,31 with a percent variation or coefficient of variation of 0.16 between multiple effective Valsalva maneuvers, 0.21 for interobserver variability, and 0.219 for a test-retest series at an average interval of 46 days. Intraclass correlations were between 0.75 and 0.98, indicating excellent agreement.31
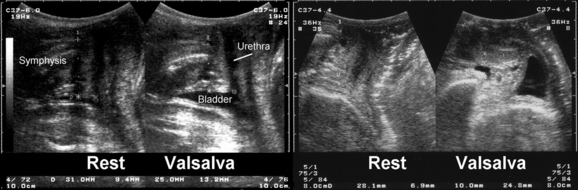
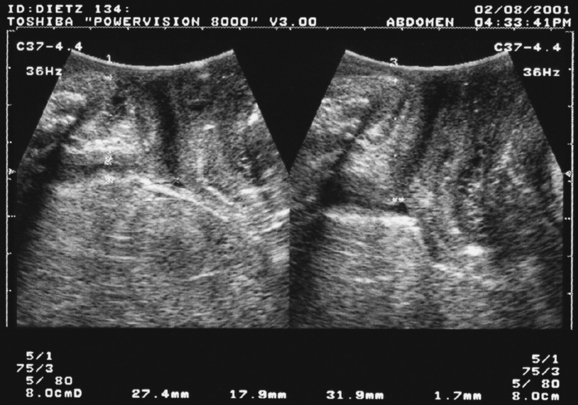
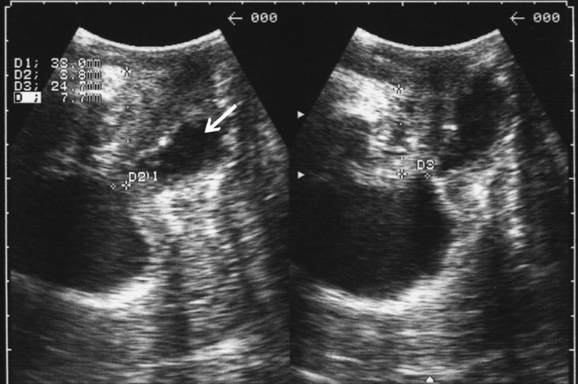
There is no definition of normal for bladder neck descent, although cutoffs of 20 and 25 mm have been proposed to define hypermobility. Average measurements in stress-incontinent women are consistently around 30 mm (HP Dietz, unpublished data). Figure 9-4 shows a relatively immobile bladder neck before a first delivery and a marked increase in bladder neck mobility after childbirth. Figure 9-5 demonstrates typical ultrasound findings in a stress-incontinent patient with a first-degree cystourethrocele, 25.5 mm of bladder neck descent, and funneling. Bladder filling, patient position, and catheterization influence measurements,25,26,32,33 and it sometimes is difficult to obtain an effective Valsalva maneuver, especially in nulliparous women. Perhaps not surprisingly, publications have presented widely different reference measurements in nulliparous women. Although two series documented mean or median bladder neck descent of only 5.1 mm34 and 5.3 mm35 in continent, nulliparous women, another study of 39 continent, nulliparous volunteers measured an average bladder neck descent of 15 mm.36 The author has obtained bladder neck descent measurements of 1.2 to 40.2 mm (mean, 17.3 mm) in a group of 106 stress-continent, nulligravid women between the ages of 18 and 23 years.37 It is likely that methodologic differences, such as patient position, bladder filling, and quality of the Valsalva maneuver (i.e., controlling for confounders such as concomitant levator activation), account for the measurement discrepancies, with all known confounders tending to reduce descent. Attempts at standardizing Valsalva maneuvers38,39 have not found widespread application because this requires intra-abdominal pressure measurement (i.e., use of a rectal balloon catheter). Other methods, such as the use of a spirometer, are likely to lead to suboptimal Valsalva maneuvers; the pressures used in the one study describing the use of such a device38 were clearly insufficient to achieve maximal or even near-maximal descent.39
The cause of increased bladder neck descent is likely to be multifactorial. The wide range of values obtained in young, nulliparous women suggests a congenital component, and a twin study has confirmed a high degree of heritability for anterior vaginal wall mobility.44 Vaginal childbirth45–47 is probably the most significant environmental factor (see Fig. 9-4), with a long second stage of labor and vaginal operative delivery associated with increased postpartum descent of the bladder neck.47 This association between increased bladder descent and vaginal parity is also evident in older women with symptoms of pelvic floor dysfunction.48 While the pelvic floor is undoubtedly affected by labor and delivery, it has been speculated that progress in labor may not be independent of pelvic floor biomechanics.49 Anterior vaginal wall mobility during a Valsalva maneuver was found to be a potential predictor of progress in labor in two independent studies.50,51
Funneling
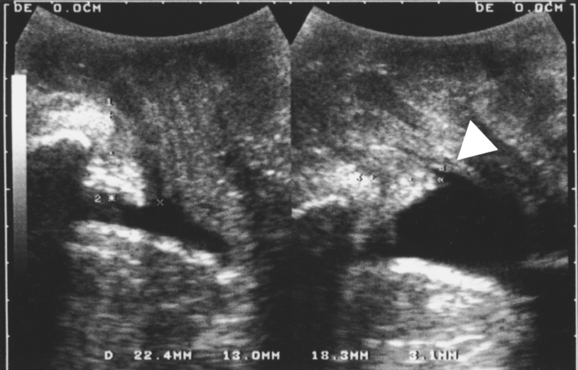
In patients with stress incontinence and in asymptomatic women,40 funneling of the internal urethral meatus may be observed during a Valsalva maneuver (see Fig. 9-5) and sometimes even at rest. Funneling is often associated with leakage. Other indirect signs of urine leakage on B-mode real-time imaging are weak gray-scale echoes (i.e., streaming) and the appearance of two linear echoes defining the lumen of a fluid-filled urethra. However, funneling may also be observed in patients with urge incontinence, and it cannot be used to prove urodynamic stress incontinence. Its anatomic basis is unclear, but marked funneling is associated with poor urethral closure pressures.41,42
Classifications developed for the evaluation of radiologic imaging43 can be modified for ultrasound; however, this approach is not generally accepted. The most common finding in cases of bladder neck hypermobility is a so-called rotational descent of the internal meatus (i.e., proximal urethra and trigone rotate around the symphysis pubis in a posteroinferior direction). In these cases, the retrovesical angle opens to up to 160 to 180 degrees from a normal value of 90 to 120 degrees, and the change in the retrovesical angle usually is associated with funneling. Often, there seems to be increased mobility of the entire urethra. A cystocele with intact retrovesical angle (90 to 120 degrees) is frequently seen in continent patients with prolapse (Fig. 9-6), and distal and central urethral fixation to the pubic rami usually seems to be relatively normal, resulting in urethral kinking. It has been surmised that this configuration distinguishes a central from a lateral defect of the endopelvic fascia,16 although proof for this hypothesis is lacking. Marked urethral kinking in these patients may protect against stress incontinence but can lead to voiding dysfunction and urinary retention. Occult stress incontinence may be unmasked once a successful prolapse repair prevents urethral kinking.
Color Doppler
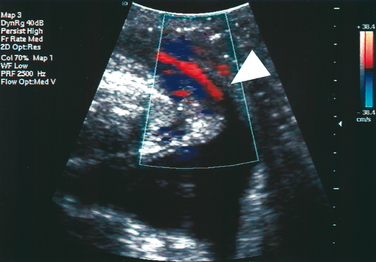
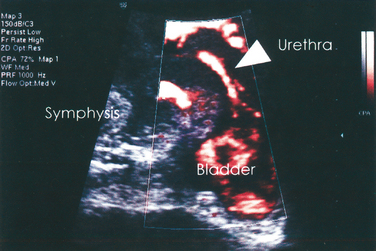
Color Doppler ultrasound has been used to demonstrate urine leakage through the urethra during a Valsalva maneuver or coughing.58 Agreement between color Doppler and fluoroscopy results was high in a controlled group with indwelling catheters and identical bladder volumes.59 Color Doppler ultrasound velocity (Fig. 9-7) and energy mapping (Color Doppler or power Doppler) (Fig. 9-8) were able to document leakage. Color Doppler ultrasound velocity was slightly more likely to show a positive result, probably because of its better motion discrimination. This results in less flash artifact and better orientation, particularly on coughing, although imaging quality depends on the systems used and selected color Doppler settings. As a result, routine sonographic documentation of stress incontinence during urodynamic testing has become feasible. Color Doppler imaging may also facilitate documentation of leak point pressures.60 Whether this is desired depends on the clinician’s preferences, because it may be argued that urine leakage and leak point pressures can be determined more easily with other methods.
Bladder Wall Thickness
There has been considerable interest in the quantification of bladder wall thickness by transvaginal or translabial ultrasound.61,62 Measurements are obtained after bladder emptying, and they are acquired perpendicular to the mucosa (Fig. 9-9). In the original description, three sites were assessed—anterior wall, trigone, and dome of the bladder—and the mean of all three was calculated. A bladder wall thickness of more than 5 mm seems to be associated with detrusor instability,61,63 although this has been disputed.64 Increased bladder wall thickness is likely caused by hypertrophy of the detrusor muscle,64 which is most evident at the dome; this may be the cause of symptoms or the effect of an underlying abnormality. Although bladder wall thickness on its own seems only moderately predictive of detrusor instability and is not in itself a useful diagnostic test, the method may be of value when combined with symptoms of the overactive bladder.65 It remains to be seen whether determination of this parameter can contribute to the workup of a patient with pelvic floor and bladder dysfunction, such as serving as a predictor of postoperative voiding function or de novo or worsened detrusor overactivity.
Levator Activity
Perineal ultrasound has been used for the quantification of pelvic floor muscle function in women with stress incontinence and in continent controls66 before and after childbirth.67,68 A cranioventral shift of pelvic organs imaged in a sagittal midline orientation is taken as evidence of a levator contraction. The resulting displacement of the internal urethral meatus is measured relative to the inferoposterior symphyseal margin (Fig. 9-10). In this way, pelvic floor activity is assessed at the bladder neck, where its effect as part of the continence mechanism is most likely to be relevant.69 Another means of quantifying levator activity is to measure reduction of the levator hiatus in the midsagittal plane or to determine the changing angle of the hiatal plane relative to the symphyseal axis. The method can also be used for pelvic floor muscle exercise teaching by providing visual biofeedback.70 The technique has helped validate the concept of the knack, a reflex levator contraction immediately before increases in intra-abdominal pressure, such as those resulting from coughing.71 Good correlations have been found between cranioventral shift of the bladder neck and palpation or perineometry.72
Prolapse Quantification
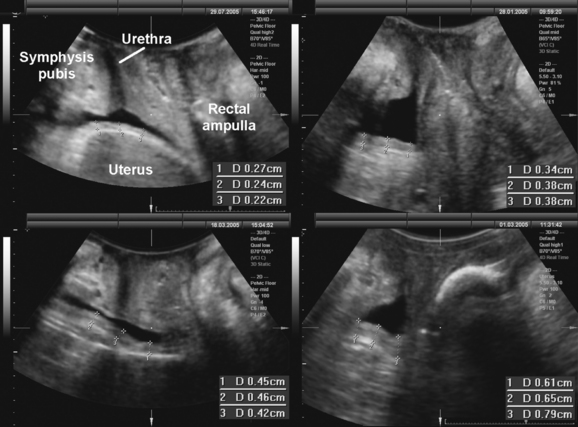
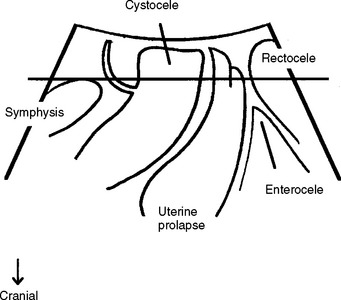
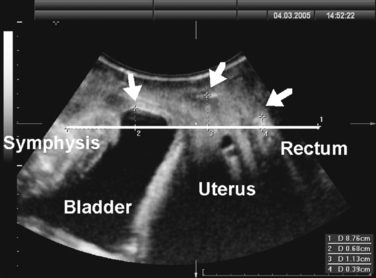
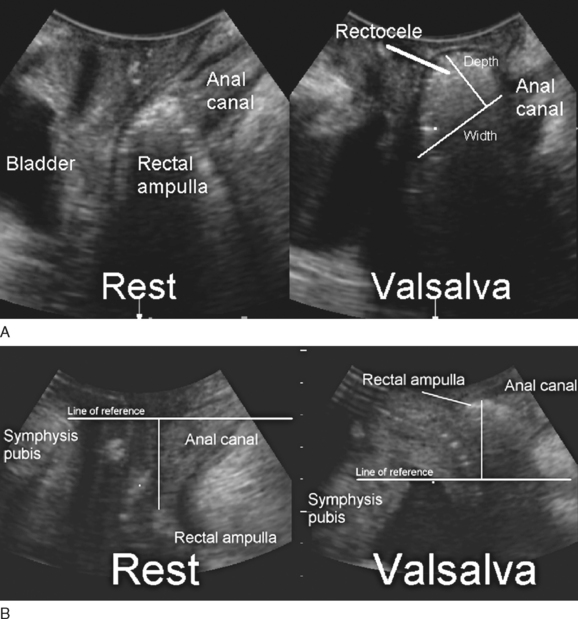
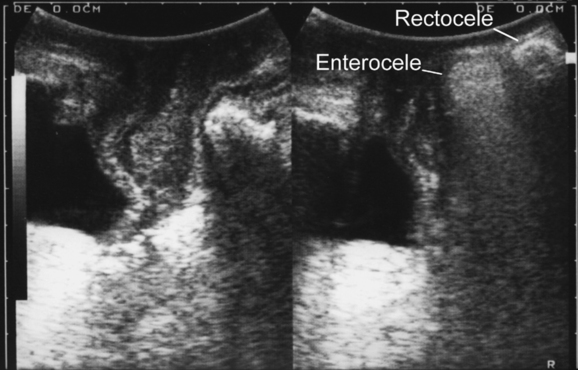
Translabial ultrasound can demonstrate uterovaginal prolapse.73,74 The inferior margin of the symphysis pubis serves as a convenient (if arbitrary) line of reference against which the maximal descent of the bladder, uterus, cul-de-sac, and rectal ampulla during a Valsalva maneuver can be measured (Fig. 9-11). On Valsalva the transducer is withdrawn to allow full development of the prolapse, while retaining contact with the insonated tissues. Angling of the transducer should be avoided in order to prevent changes in the relative position of transducer and symphysea axes. Figure 9-12 shows a three-compartment prolapse, with the uterus leading. Findings have been compared with clinical staging and the results of a standardized assessment according to criteria developed by the International Continence Society,75 with good correlations shown for the anterior and central compartments.76 Although there may be poorer correlation between posterior compartment clinical assessment and ultrasound, not the least due to variable rectal filling, it is possible to distinguish between true rectocele (i.e., defect of the rectovaginal septum) (Fig. 9-13A) and perineal hypermobility without fascial defects (see Fig. 9-13B). True rectoceles may be present in young, nulliparous women but are more common in parous women. In some instances, they arise in childbirth.77 From imaging experience, fascial defects seem to almost always be found in the same area (i.e., very close to the anorectal junction), and they most commonly are transverse. Many are asymptomatic. Routine posterior repair often results in reduction or distortion of such defects without effecting closure.
The ability to differentiate different forms of posterior compartment descent should allow better surgical management in the future, especially because enterocele (Fig. 9-14) can easily be distinguished from rectocele. It appears that colorectal surgeons are starting to use the technique to complement or replace defecography,78 and perineal ultrasound can also be used for exoanal imaging of the anal sphincter.79,80
Disadvantages of the method include incomplete imaging of bladder neck, cervix, and vault with large rectoceles and possible underestimation of severe prolapse due to transducer pressure. Occasionally, apparent anterior vaginal wall prolapse turns out to be caused by a urethral diverticulum81,82 (Fig. 9-15) or a paravaginal cyst.
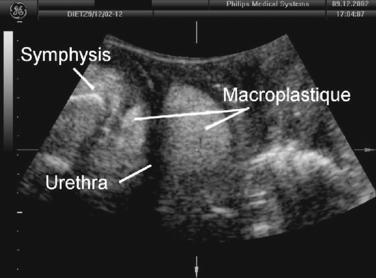
The main use of this technique may prove to be in outcome assessment after prolapse and incontinence surgery for clinical and research applications. Elevation and distortion of the bladder neck arising from a colposuspension is easily documented.83,84 Fascial and synthetic slings are visible posterior to the trigone or the urethra (Figs. 9-16 and 9-17). Bulking agents such as Macroplastique (Fig. 9-18) show up anterior, lateral, and posterior to the proximal urethra. It has been demonstrated that overelevation of the bladder neck on colposuspension is unnecessary for cure of urodynamic stress incontinence, and elevation may also have a bearing on postoperative symptoms of voiding dysfunction and de novo detrusor instability.83,84
Implants
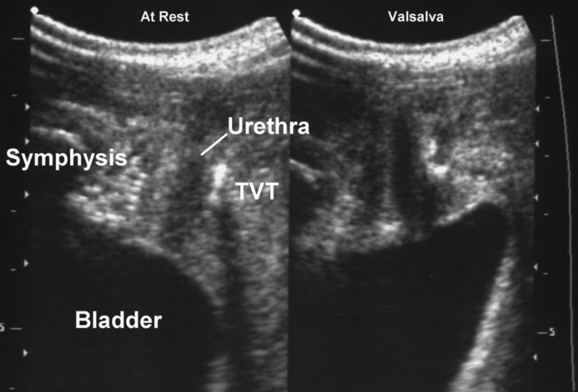
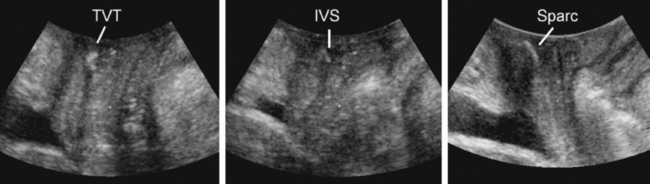
Ultrasound has contributed significantly to the investigation of new surgical procedures, such as wide-weave suburethral Prolene slings, showing that they act by urethral kinking or dynamic compression against the posterior surface of the symphysis pubis.85 Available synthetic slings are easily visualized posterior to the urethra86–93 (see Fig. 9-16). Wide-weave monofilament mesh such as tension-free vaginal tape (e.g., Gynecare TVT), SPARC sling, and Monarc Subfascial Hammock or transobturator (TOT) sling are more echogenic than more tightly woven multifilament implants, such as the IVS (i.e., polypropylene mesh) (see Fig. 9-17), but virtually all can be identified and followed in their course from the pubic rami laterally to the urethra centrally. The difference between transobturator tapes (e.g., Monarc, TOT) and tapes placed through the space of Retzius (e.g., TVT, SPARC, IVS) is evident when following the tapes in the parasagittal or axial planes. In the parasagittal plane, transobturator tapes often can be seen to perforate the obturator fascia and muscle close to the insertion of the pubovisceral muscle; sometimes, they traverse the most inferomedial component of the levator before exiting the pelvis.93 Wide-weave mesh implants used in procedures, such as the Perigee, Apogee or Prolift implants, are very echogenic and easily identified,94 and their transobturator or pararectal extensions can be followed for some distance, although 3D or 4D imaging allows much more comprehensive evaluation.
Ultrasound has demonstrated the wide margin of safety and efficacy of suburethral tapes in regard to placement (which helps explain their extraordinary success) and allayed concerns regarding tape shrinkage and tightening due to scar formation.90,91 The assessment of bladder neck mobility before implantation of a suburethral sling may predict success or failure,95 an observation that makes perfect sense considering that dynamic compression relies on relative movement of implant and native tissues.
Paravaginal Defect Imaging
Transabdominal ultrasound has been used to demonstrate lateral defects of the endopelvic fascia, also called paravaginal defects. However, this method has not been fully validated, and a prospective study showed poor correlation with clinically observed defects.96 Several factors may limit the predictive value of transabdominal ultrasound in the identification of paravaginal defects: the poor definition of an optimal scanning plane, the influence of uterine prolapse or a full rectum, and the inability to observe the effect of a Valsalva maneuver (which would dislodge the transducer) by transabdominal imaging. It is likely that levator trauma (see below) is frequently misinterpreted as a “paravaginal defect.” Fascial trauma is highly likely in patients with a full avulsion, but it is conceivable that fascial defects may occur in women with intact muscle. Much work remains to be done in this field.
Other Findings
A range of other abnormalities, incidental or expected, may sometimes be detected on translabial ultrasound, although a full pelvic ultrasound assessment does require a transvaginal approach. Urethral diverticula (see Fig. 9-15),78,97 labial cysts, Gartner’s duct cysts (Fig. 9-19), or bladder tumors (Fig. 9-20) may be identified, and intravesical stents and bladder diverticula also can be visualized.16 Postoperative hematomas may be visible after vaginal surgery or TVT placement and sometimes explain clinical symptoms such as voiding dysfunction or persistent pain (Fig. 9-21).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree