Pathology of the Renal Pelvis and Ureter
Brett Delahunt
Hemamali Samaratunga
The renal pelvis and ureter are tubular structures that facilitate passage of the urine from the kidney to the urinary bladder. Despite their relatively simple architecture, these organs are subject to a wide variety of pathologic processes. In particular, the complex nature of nephro-ureterogenesis accounts for the observed diversity of congenital abnormalities seen at this site, which may lead to urinary reflux, urinary tract infections, and ultimately renal failure. In addition to these congenital abnormalities, chronic exposure to toxins and metabolites within the urine may promote the development of clinically important diseases such as lithiasis, and mucosal metaplasia and neoplasia. Although a number of reported pelviureteral disorders are rarely encountered in clinical practice, many of the more common conditions account for a significant morbidity and mortality in both pediatric and adult populations.
ANATOMY AND HISTOLOGY
The renal pelvis and ureter are fibromuscular tubes lined by mucosa. The ureter is 30 cm long with an average diameter of 5 mm. There are narrowings of the lumen at the ureteropelvic junction where the external and common iliac vessels cross and where the ureter enters the bladder. These are natural sites for obstruction and impaction of stones and are frequently the sites where pathology is observed.1
Urothelium lines the complete length of the renal pelvis and ureter and varies in thickness from two to three cells in the renal pelvis to four to six cells in the ureter. The surface of the urothelium consists of larger cells aligned parallel to the surface (umbrella cells). These have eosinophilic cytoplasm and occasional binucleate forms and mucin-filled vacuoles are seen.2 The surface of the superficial layer is covered by an impervious trilaminar membrane, which is convoluted in the resting nondistended state.3 Desmosomes are present between cells of the superficial layer and between superficial and intermediate cells. Beneath the superficial layer, cells are aligned perpendicular to the basement membrane, and in thickened epithelium a condensed basal cell layer may be present. Scattered glycogen-filled vacuoles are often present within the deeper layers. It has been demonstrated that the nuclei of cells of the renal pelvic urothelium are larger than those of the bladder. As a consequence of this, caution is required in interpreting dysplasia and carcinoma in situ (CIS) in frozen sections of ureter.
The lamina propria consists of vascular fibrous tissue, which is more condensed toward the deep aspect.1 Scattered elastin fibers are present, which cause folding of the mucosa in the resting state. Beneath the submucosa, bands of smooth muscle form the muscularis propria, which increases in thickness distally (Fig. 4-1). This is arranged into an inner longitudinal layer and an outer circular layer, although this division is not discernible within the renal pelvis and proximal ureter. The passage of urine through the renal pelvis and ureter is facilitated by peristalsis, with an action potential being propagated between myocytes by numerous close contacts or nexuses.1 In the renal pelvis the fibers of the muscularis have a spiral arrangement, which extends into the immediate proximal ureter.4 This spiral arrangement gives rise to a fragmented appearance to the muscularis in histologic sections, and this has been considered, erroneously, to be a diagnostic feature of ureteropelvic junction obstruction.
In the renal pelvis the muscularis propria is covered by fat, which blends into the fat of the renal hilum.2 The adventitia of the proximal ureter consists of loose connective tissue containing collagen, fibroblasts, myocytes, and nerve fibers. This is condensed and thickened in the distal ureter to form Waldeyer fascia, which continues as the adventitia of the bladder.
EMBRYOLOGY
The urinary tract develops from intermediate mesoderm, and three separate renal structures form sequentially. Initially the transient pronephros is formed with an associated nephrogenic duct, which opens into the cloaca. With the development of this structure into the mesonephros by week 4, the nephrogenic duct forms the mesonephric duct from which the ureteric bud develops on its dorsomedial surface. The bud elongates caudally and its junction with the mesonephric
duct migrates toward the cloaca to form separate ureteric and Wolffian ducts by week 6.5 The caudal end of the ureteric duct forms the ampulla, and this fuses with the metanephric mesenchyme. This fusion induces branching of the ampullary bud to form the renal pelvis, calyces, and collecting ducts, and also induces differentiation of metanephric mesenchyme into nephrons and supporting stroma.6,7 By week 12 the ureteral muscularis develops, and by week 14 there is recognizable urothelium lining the upper tract.8
duct migrates toward the cloaca to form separate ureteric and Wolffian ducts by week 6.5 The caudal end of the ureteric duct forms the ampulla, and this fuses with the metanephric mesenchyme. This fusion induces branching of the ampullary bud to form the renal pelvis, calyces, and collecting ducts, and also induces differentiation of metanephric mesenchyme into nephrons and supporting stroma.6,7 By week 12 the ureteral muscularis develops, and by week 14 there is recognizable urothelium lining the upper tract.8
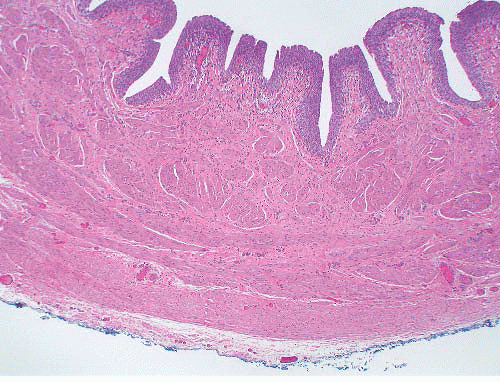 FIGURE 4-1 ▪ Distal ureter showing prominence of the inner longitudinal and outer circular layers of the muscularis propria. |
It has been claimed that the ureter becomes temporarily obliterated during week 6 and recanalization commences in the midportion and extends both proximally and distally.9 This process of obliteration and recanalization has been questioned, and it has been suggested that this is merely a collapse of the ureteral lumen prior to the onset of urine output by the metanephros.10 There is a further temporary obstruction of the ureter by the Chawalla membrane that forms as a thin band of epithelial cells across the ureteral orifice during week 6.11 Persistence of luminal obliteration or of the Chawalla membrane has been implicated as a cause of ureteral valves and ureteral stenosis.
MALFORMATIONS
Agenesis, Duplication, and Ectopia
Renal agenesis and duplication, and positional abnormalities of the upper tract are the result of growth failure or abnormal branching of the ureteric duct from the mesonephric duct.
Total failure of the ureteric duct to develop results in renal agenesis. Bilateral renal agenesis is rare, with a reported incidence of 3.5/105 live births,12,13 while unilateral agenesis is more frequently encountered12 and in one series was detected in 1/1,200 of children on screening.14 Renal agenesis may also arise when there is failure of the ureteric duct to fuse with the metanephros, and where the ureteric duct may persist to form a blind diverticulum part way along the length of the ureter.
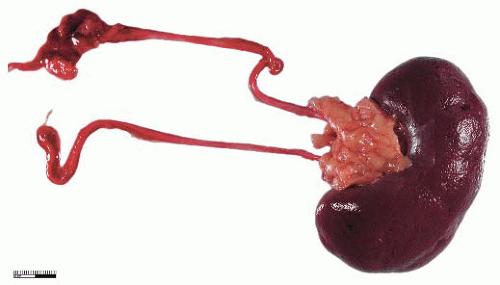
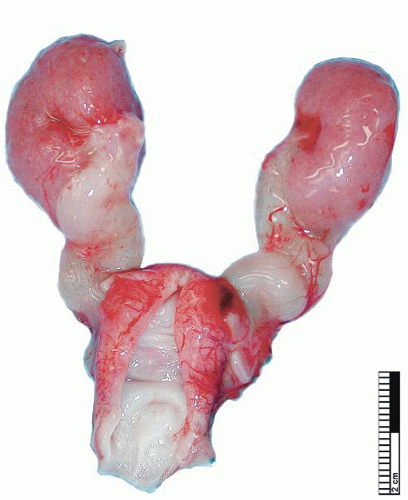
During elongation of the ureteric duct, with associated renal ascent, splitting of the ampullary bud may occur. In cases where this splitting occurs early in nephrogenesis or if two separate ampullary buds form along the length of the mesonephric duct, then double renal pelves and ureters will form, giving rise to a duplex collecting system (Fig. 4-2). Typically the vesicoureteral orifice of the upper pole ureter in a duplex system lies medial and caudal to the lower pole orifice, although exceptions to this rule have been reported. When splitting of the ureteric duct is a late event, a bifid ureter forms with two renal pelves, which drain into separate ureters that fuse to terminate in a single vesicoureteral orifice15,16 (Fig. 4-3). The site of the junction of bifid ureters depends on timing of the branching of the ureteric
duct; however, in the majority of cases, this is in the lower third of the ureter and may be sited in the bladder wall.17
duct; however, in the majority of cases, this is in the lower third of the ureter and may be sited in the bladder wall.17
 FIGURE 4-3 ▪ Double renal pelves and ureters fusing superior to the vesicoureteric orifice to form a single ureter. |
During embryogenesis the developing ureteric duct migrates distally along the mesonephric duct to fuse with the bladder, and the vesicoureteral orifice migrates to its normal trigonal position. If the ureteric duct develops in close proximity to the bladder, then migration of the vesicoureteral orifice extends beyond the trigone in both the caudal and lateral planes. Often the ectopic ureter is inserted more directly into the bladder wall with loss of the valve mechanism and resulting vesicoureteral reflux.18 In extreme cases the ureteral orifice extends beyond the bladder, and the ureter terminates in the urethra or the genital structures that develop from the mesonephric duct.16 In females ureteral ectopia is associated with a duplex system in 80% of cases, while in males there is usually a single renal outflow tract.18 Further, as the ureteric duct takes its origin from the mesonephric duct, upper tract abnormalities are often associated with other malformations of the urogenital system and are found in the prune belly and VATER syndromes and trisomy 21.
Clinical features of congenital abnormalities of the upper tract are variable although there is often reflux and urinary stasis leading to urinary tract infection, lithiasis, and fistula formation. Ureteral duplications are often asymptomatic although cyclic abdominal pain may occasionally be a presenting feature.17 Ultrasonography is valuable in the demonstration of malformations in utero and in neonates. The role of the pathologist may be to define the anatomic basis of a functional deficiency.
Ureteropelvic Junction Obstruction
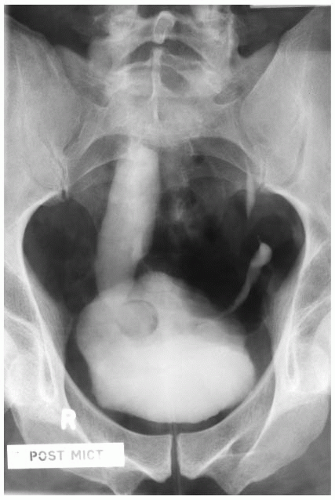
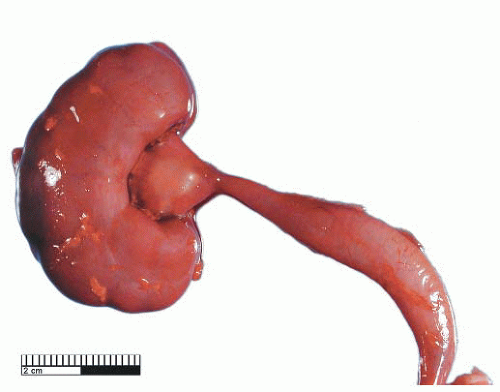
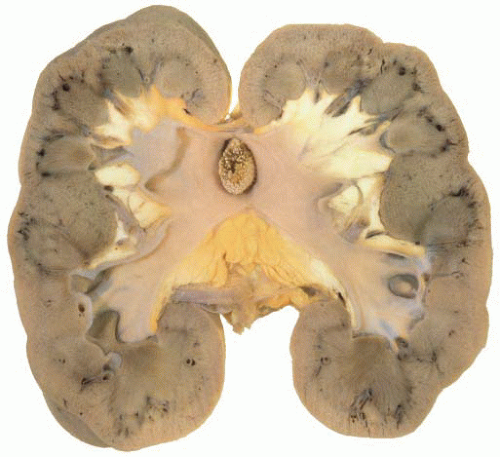
Ureteropelvic Junction (UPJ) obstruction refers to the significant functional impairment of urinary flow from the renal pelvis to the ureter leading to hydronephrosis (Fig. 4-4). The majority of cases are congenital and occur in the pediatric age group. More rarely the obstruction may be secondary to postoperative or inflammatory strictures, renal stones, and urothelial neoplasms, including fibroepithelial polyps.19,20 Rarely UPJ obstruction is associated with renal parenchymal neoplasia. There is also a reported association with congenital renal abnormalities in 15% to 20% of patients,21 with agenesis and cystic renal dysplasia in the contralateral kidney,22 and with vasculitis.23 Functional obstruction has also been reported due to extrinsic compression by an aberrant lower pole vessel. This is seen in 16% to 20% of patients with UPJ obstruction with a median age of 67 months at presentation.24 UPJ obstruction is more likely to be unilateral in adults, while pediatric cases are more frequently bilateral.
Congenital UPJ obstruction affects 13,000 newborns annually in the United States with hospitalization rates of 2.4/100,000 for patients aged ≤18 years. The highest incidence is in children <3 years of age with hospital admission rates of 9.3/100,000 being reported,19 and in this age group there is a male predominance.25 Ethnicity appears not to be a significant factor although slightly higher hospitalization rates for UPJ obstruction have been noted for patients of Hispanic origin.19
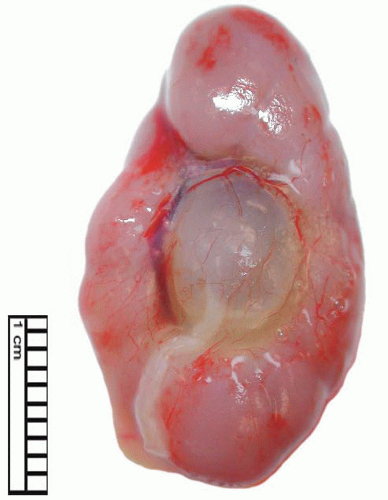 FIGURE 4-4 ▪ Ureteropelvic junction obstruction. The renal pelvis is distended proximal to the UPJ obstruction. |
Transport of urine from the renal papilla to the bladder is active and dependent on smooth muscle contraction, with some modulation by the autonomic nervous system.26 In many cases of clinical UPJ obstruction, no identifiable lesion is seen and the impairment is functional.27 Earlier studies have demonstrated abnormalities at both a cellular and ultrastructural level, and in particular increased interstitial collagen has been implicated,28 although this is likely to be a secondary effect. Folds in the muscle or overlying mucosa, or the development of kinks or strictures that lead to reorientation of muscle bundles into a predominantly longitudinal pattern, have also been suggested as a pathogenic mechanism for UPJ obstruction.29,30 Recent reports utilizing S100 protein, CKIT protooncogene protein (CD117), and synaptophysin immunohistochemistry suggest a defect in innervation as the cause of UPJ obstruction.31 The UPJ is not normally innervated, and in cases of obstruction it is apparent that this segment extends for a longer distance down the proximal ureter.32 Defective innervation is supported by a finding of increased vasoactive intestinal peptide and decreased levels of synaptophysin and nerve growth factor receptor in UPJ obstruction.32 Transforming growth factor beta 1 (TGF-β1) has recently been shown to be increased in UPJ obstruction; however, this may be a secondary effect leading to the promotion of extracellular matrix formation and collagen synthesis.33
Prior to the advent of ultrasound investigations, UPJ obstruction was usually diagnosed during investigations for
azotemia, urinary tract infection, or hematuria. The majority of cases are now discovered by the detection of renal pelvis dilatation on prenatal or perinatal ultrasound, and it is recommended that a dilatation of ≥5 mm be further investigated.34 Asymptomatic cases of UPJ obstruction are usually treated conservatively, and in those patients with good renal function, the outcome is favorable whether the treatment is conservative or surgical. While patients with moderate impairment may benefit from pyeloplasty, those with poor function usually have minimal recovery following pyeloplasty.27
azotemia, urinary tract infection, or hematuria. The majority of cases are now discovered by the detection of renal pelvis dilatation on prenatal or perinatal ultrasound, and it is recommended that a dilatation of ≥5 mm be further investigated.34 Asymptomatic cases of UPJ obstruction are usually treated conservatively, and in those patients with good renal function, the outcome is favorable whether the treatment is conservative or surgical. While patients with moderate impairment may benefit from pyeloplasty, those with poor function usually have minimal recovery following pyeloplasty.27
The gross surgical specimen in congenital UPJ obstruction is usually funnel shaped (Fig. 4-5), and thickening of the portion of the renal pelvis in the vicinity of the UPJ may be present. The histologic findings are variable and nonspecific, and occasionally no abnormality is seen. The mucosa is usually normal although projections resembling mucosal folds may be present.35 There is occasionally hypertrophy of the smooth muscle layer32 although this is more commonly thinned, and segmental loss of smooth muscle has also been reported.36 Interstitial fibrosis is the most common finding although this is variable and may be associated with a mild diffuse chronic inflammatory infiltrate (Fig. 4-6). On electron microscopy, increased interstitial collagen is usually observed.
Ureteral and Paraureteral Diverticulum
Ureteral diverticula are rare and may be congenital or acquired. Symptoms are nonspecific; however, dysuria and hematuria may be presenting features.37 Congenital diverticula are related to sites of weakness within the ureteral wall and are most commonly found adjacent to the ureteropelvic and vesicoureteral junctions. Other abnormalities of the urinary tract may be found in conjunction with congenital diverticula.38
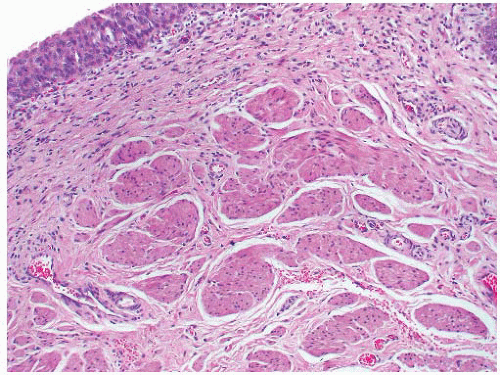 FIGURE 4-6 ▪ Ureteropelvic junction obstruction. The distal renal pelvis shows marked interstitial fibrosis. |
Acquired diverticula are probably secondary to chronic infection or ureteral obstruction.39,40 Occasionally multiple diverticula may be present, and it has been suggested that these are acquired, being the result of chronic infection.41 It is recommended that treatment of small acquired diverticula should be directed toward management of the predisposing condition,36 although larger diverticula may require surgical resection.
Histologically, congenital diverticula contain all three layers of the ureteral wall, while acquired diverticula usually have a thinned mucosal layer.37
Paraureteral diverticula originate within the bladder wall, adjacent to the ureteral orifice, and are due to a failure of normal muscle development or a defect in Waldeyer fascia.42 In some cases, these can expand to involve the ureter with vesicoureteral reflux or outflow obstruction.43 There is occasionally coexisting renal dysplasia, and an association with pelvicalyceal duplication has been reported.44,45 Treatment is surgical and consists of diverticulectomy with or without ureteral reimplantation.43
Ureterocele
Ureterocele is the cystic dilatation of the distal ureter that projects into the bladder (Fig. 4-7). This most commonly occurs in Caucasian females,46 and there is an annual hospitalization rate of 1/105 in the pediatric age group, with 92% of hospital admissions being children under the age of 2 years.47 The pathogenesis is uncertain and persistence of Chawalla membrane has been suggested, as has abnormality of the ureteral muscularis.48 It has been estimated that there is an association with a duplex system in 95% of cases in females and 44% of cases in males.47 Ureterocele associated with duplex ureter usually occurs in the upper pole ureter and is situated more inferiorly than ureterocele that arises from a single ureter. Previously ureteroceles arising in association with duplex ureters have been designated ectopic ureterocele; however, this term is now limited to those
ureteroceles that extend beyond the bladder into the bladder neck or urethra.49 If a ureterocele burrows between the bladder mucosa and muscularis to lie intramurally, the term cecoureterocele is applied.
ureteroceles that extend beyond the bladder into the bladder neck or urethra.49 If a ureterocele burrows between the bladder mucosa and muscularis to lie intramurally, the term cecoureterocele is applied.
Ureterocele is associated with dilatation of the proximal ureter and often results in reflux and hydronephrosis. Larger ureteroceles may also obstruct the contralateral ureter or the normal ipsilateral ureter in a duplex system. Obstruction of renal outflow from ureterocele may lead to cystic renal dysplasia or renal scarring, and in 10% of cases hypertension develops.50
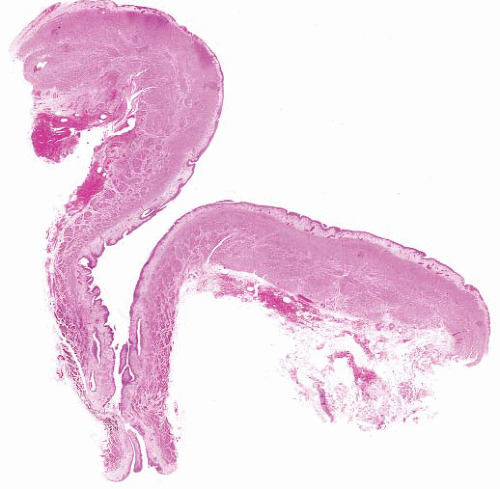
The histologic features of ureterocele relate to chronicity. The surface mucosa of ureterocele originates from the bladder, while the cyst lumen is lined by ureteral mucosa51 (Fig. 4-8). The muscularis may show hypertrophy or be attenuated and atrophic, with interstitial edema and variable interstitial fibrosis.
Treatment of ureterocele consists of early prophylactic antibiotic therapy, to minimize the effect of urinary tract infection. Endoscopic puncture may be undertaken as an emergency procedure, followed by surgical excision of the ureterocele and ureteral reconstruction.52
Megaureter
Megaureter is dilation of the ureter. This is considered to be primary if it is the result of a defect of intrinsic smooth muscle. It is most commonly seen in the pediatric age group and is characterized by a ureteral diameter of >7 mm. Megaureter is not a specific diagnosis and may be associated with reflux or outflow obstruction, although a significant proportion is nonrefluxing and nonobstructed. These three categories of megaureter are further subdivided into primary and secondary groups53 (Table 4-1). The pathogenesis of megaureter is dependent on the type; however, there is an association with abnormalities of the urinary tract, particularly cystic renal dysplasia.54
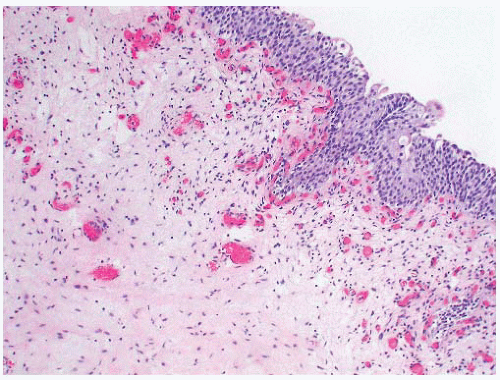 FIGURE 4-8 ▪ Ureterocele. There is hyperplasia of the urothelium with edema, telangiectasia, and chronic inflammation of the grossly thickened lamina propria. |
Refluxing Megaureter
Refluxing megaureter is characterized by retrograde flow from the bladder into the ureter. This may be unilateral or bilateral and is more common in females.55 Primary refluxing megaureter results from abnormalities of the vesicoureteral junction, associated with loss of the valve function of the intramural ureteral segment.54 The histologic features are
nonspecific with mural thickening, loss of smooth muscle, and an increase in interstitial connective tissue. Widespread collagen deposition is seen, and quantitative studies have shown a predominance of type III (juvenile) collagen.56 Treatment is dependent on the severity of the reflux and degree of abnormality of the intravesical ureter. In severe cases early reimplantation is required, and in mild cases reflux may cease as the child grows. Secondary refluxing megaureter may be associated with urethral obstruction, neurogenic bladder, prune belly syndrome, and megacystis.53,57
nonspecific with mural thickening, loss of smooth muscle, and an increase in interstitial connective tissue. Widespread collagen deposition is seen, and quantitative studies have shown a predominance of type III (juvenile) collagen.56 Treatment is dependent on the severity of the reflux and degree of abnormality of the intravesical ureter. In severe cases early reimplantation is required, and in mild cases reflux may cease as the child grows. Secondary refluxing megaureter may be associated with urethral obstruction, neurogenic bladder, prune belly syndrome, and megacystis.53,57
Table 4-1 ▪ CLASSIFICATION OF MEGAURETER | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Obstructed Megaureter
Primary obstructed megaureter results from the presence of an aperistaltic ureteral segment situated immediately proximal to the vesicoureteral junction58 (Fig. 4-9). Ureteral obstruction in these cases is functional as no stricture is seen, and abnormalities of neuromuscular transmission, resulting in an adynamic segment, have been implicated. Histologic findings are nonspecific, and there is often muscle hypoplasia with disorganization of myofibrils. Increased amounts of collagen are present, and in particular, an increase in collagen types I and III has been noted.56 Hyperplasia of the ureteral muscularis, associated with ectopic insertion,59 as well as total loss of the muscularis,60 have been described. Obstructed megaureter is treated by excision of the lower stenotic segment and ureteral reimplantation.53
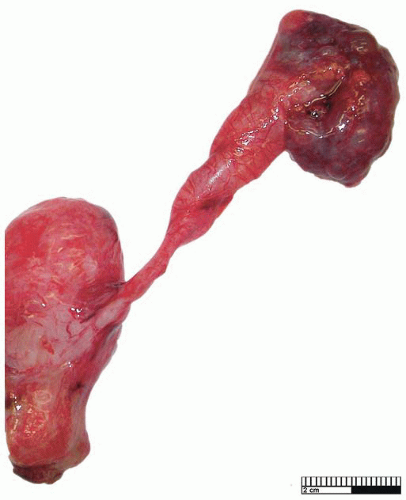 FIGURE 4-9 ▪ Primary obstructed megaureter with ureter of normal caliber adjacent to the vesicoureteral junction. |
Nonobstructed, Nonrefluxing Megaureter
In the majority of cases of perinatal megaureter, there is no evidence of either ureteral obstruction or reflux.
The pathogenesis of nonobstructed, nonrefluxing megaureter is unknown although abnormal ureteral compliance or persistence of fetal ureteral architecture, with transient obstruction, have been proposed.61,62 This is confined to the distal ureter immediately proximal to the vesicoureteric junction with ureteral dilatation proximal to this,57 and the dilated segment of the ureter is often fusiform and lacks the tortuosity associated with other forms of megaureter (Fig. 4-11). There is overlap with the histology of obstructed megaureter with interstitial fibrosis, fibrosis of the periureteral sheath, and hypoplasia of the inner (longitudinal) muscle layer being reported.63
Management of nonobstructed, nonrefluxing megaureter is usually expectant in the absence of urinary tract infection or loss of renal function. In severe cases, with declining renal function, ureteral reimplantation is undertaken.57
Secondary causes of nonobstructed, nonrefluxing megaureter may result from conditions leading to polyuria and from bacterial toxins associated with urinary tract infection.53
Ureteral Dysplasia
Rarely congenital nonrefluxing megaureter is associated with ureteral dysplasia where the muscularis has poorly formed myocytes, with absent or poor organization into muscle bundles. There is usually pronounced interstitial fibrosis with a variable infiltrate of lymphocytes and plasma cells.64,65 On ultrastructural examination there is thinning of myofilaments, wide separation of cell membranes, a decrease in numbers of nexuses, and an increase in collagen fibers and ground substance.65
It has been shown that ureteral dysplasia is evident at 11 weeks’ gestation, and as there is an association with urinary outflow obstruction early in nephrogenesis, cystic renal dysplasia frequently occurs.63 The pathogenesis of ureteral dysplasia is unknown although muscle contractility studies show decreased amplitude of contractions and decreased response to adrenergic drugs.66 On imaging there is dilatation of the involved ureteral segment, which is usually sited in the distal ureter. In severe cases interstitial fibrosis leads to ureteral obstruction, although, in most cases this obstruction is functional rather than anatomical.67
Treatment of ureteral dysplasia involves resection of the dysplastic segment with ureteral reimplantation.68
Ureteral Stricture
Ureteral stricture may be either congenital or secondary to trauma and inflammation. The pathogenesis of congenital stricture is uncertain and may be associated with failure of complete recanalization of the ureter, which undergoes temporary obliteration during fetal development.69,70 An alternative theory is that congenital ureteral stricture is due to external compression of the ureter by fetal vessels leading to incomplete smooth muscle development.71 This latter theory accords with the histologic findings, which consist of segmental absence of muscle and narrowing of the ureteral lumen. Congenital ureteral stricture may occur in isolation or may be a component of multiple organ syndromes.72 There is also an association with ipsilateral and contralateral cystic renal dysplasia, solitary kidney, and blind ending of the contralateral ureter.
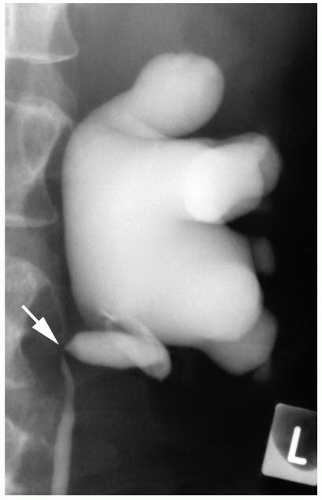 FIGURE 4-12 ▪ Ascending ureteropyelogram showing stricture and medial deviation of the upper ureter (arrow) secondary to infiltration by retroperitoneal tumor. |
Secondary ureteral stricture is a recognized complication of ureteral endoscopy and pelvic surgery, with an incidence ranging from 0.5% to 3%.73 Other secondary causes of ureteral stricture are trauma, ureteral infections, including tuberculosis and inflammation in adjacent organs, radiation, ureteral lithiasis; vasculitis and ischemia, amyloidosis, and endometriosis.74, 75, 76, 77, 78, 79
Primary tumors of the ureter, especially urothelial carcinoma, usually present with ureteral stricture, and hydronephrosis and stricture may also be seen in association with extraureteral tumors that involve the ureter by direct infiltration or metastatic spread (Fig. 4-12).
Ureteral Valves
Ureteral valves are a rare cause of ureteral outflow obstruction with <60 cases being reported.80 The majority of cases occur in males in the pediatric age group, with hydronephrosis being the most frequently associated finding. In many cases there is also an association with abnormalities of the kidney and ureters.11
The pathogenesis of ureteral valves is debated; however, it has been suggested that they represent a persistent Chawalla membrane or an exuberant form of the ureteral folds seen in normal fetal development.11,81
Specific criteria for the diagnosis of ureteral valves have been proposed; (i) The leaflets should be covered by mucosa and contain smooth muscle, (ii) There should be ureteral obstruction proximal to the fold but not distal to it, and (iii) There should be no other evidence of either functional or mechanical obstruction.81 The requirement for the identification of smooth muscle in the leaflet is debated, while others require smooth muscle in the leaflet base.11 Valves may occur throughout the length of the ureter, with 50% reported from the proximal ureter, 17% the mid ureter, and 33% the distal ureter. Histologically, ureteral valves may form leaflets or an annular ring of mucosal-covered connective tissue.
Management of valve leaflets is surgical with most treated by ureteroureterostomy, ureteropyelostomy, or longitudinal ureterotomy.11
Ureteral folds are rarely detected on excretory urography, and these occasionally have a corkscrew appearance.10 These folds consist of lamina propria and smooth muscle and probably represent persistent fetal ureteral tortuosity.10,82 Most cases of ureteral folds are not associated with outflow obstruction, although rarely hydronephrosis may be a presenting feature.83
INFLAMMATION AND INFECTION
Renal Pelvis and Ureteral Infection
Infection of the upper urinary tract is usually secondary to lower tract infection and may be associated with urinary tract obstruction, voiding dysfunction, and urinary tract abnormalities (ureteral duplication, megaureter, and UPJ obstruction). Other significant contributing factors include catheterization, instrumentalization, and urinary tract lithiasis, while ureteral infection may occur secondary to pyelonephritis.
The most common pathogenic bacterial organism in the urinary tract is Escherichia coli although Proteus, Klebsiella, Enterococcus, Pseudomonas, and Serratia are frequently encountered. Struvite lithiasis is often associated with Proteus and less commonly Morganella infection.84
Mycobacterium infection of the ureter (Fig. 4-13A and B) is usually secondary to renal infection and may result in the development of stricture leading to hydronephrosis. Mycobacterium tuberculosis is most frequently involved, although M. avium, M. kansasii, and M. bovis infections are also well described.
Upper tract fungal infections are usually associated with systemic disease or localized renal infection in an immunocompromised patient. A wide variety of fungi have been reported as renal pelvis and ureteral pathogens with Candida being the most commonly seen. This may form a fungal bezoar within the renal pelvis and, as is reported for Aspergillus and Coccidioides, may result in ureteral stricture formation.85,86 There is a single case report of extragenital granuloma inguinale presenting as a soft tissue neoplasm involving the ureter.87
Malacoplakia
While more commonly occurring in the urinary bladder, renal parenchyma, and genital tract, occasional cases of malacoplakia of the renal pelvis and ureter (Fig. 4-13C) have been reported.88, 89, 90 There is a female predominance with ages of patients ranging from 24 to 78 years (mean 66 years).88,91 In the majority of cases, there were no reported preexisting conditions although an association with concurrent acute inflammation and immunosuppression has been noted,89,92 and there is a single case report of malacoplakia in a transplant ureter.93
Malacoplakia of the renal pelvis and/or ureter usually presents as an exophytic tumor-like lesion on imaging and may be mistaken for urothelial carcinoma.94 Urinary cytology has also been shown to be diagnostic in rare instances.95 Disease may be bilateral or unilateral and is often associated with urinary obstruction and hydronephrosis.94 Malacoplakia has also been reported as a cause of secondary UPJ obstruction.96 On occasion inflammation may be limited to the renal pelvis or ureter; however, there is usually concurrent malacoplakia in the urinary bladder or renal parenchyma, and it has been suggested that involvement of the urinary outflow tract is a secondary feature.97
Grossly the lesion may be solitary or multifocal and is soft friable and exophytic ranging from white to pale yellow in color.
Histology shows typical features of malacoplakia with early lesions resembling xanthogranulomatous inflammation without Michaelis-Gutmann bodies, while later lesions may be associated with interstitial fibrosis.92,98 On ultrastructural examination both histiocytes and urothelial cells contain cytoplasmic multilamellar bodies.99
Few reports have identified organisms within foci of malacoplakia although E. coli has been isolated,86 and treatment usually involves either localized or radical resection with adjuvant antibiotic therapy. Occasionally antibiotic treatment alone, or in combination of ureteral stenting, is successful.88,89,100
Renal Pelvis and Ureter Lithiasis
Renal lithiasis is a common problem, especially in Western countries, and is increasing in frequency. Within the United States, it is estimated that 13% of males and 7% of females will be diagnosed with a renal stone and that the likelihood of recurrence is up to 50% on a 5-year follow-up.101,102 The advent of nonsurgical treatments has led to a decline in hospitalization for renal lithiasis although in 2000, 62/100,000 population were treated as inpatients for upper tract stones in the United States, with the rates being highest for the 55- to 64-year age group.101
Stone composition is variable (Table 4-2) and in the majority of instances stones form as the result of metabolic disorders, dietary factors, and/or urinary tract abnormalities, although medications such as adenosine, ephedrine, indinavir, and triamterene have been implicated. There is a strong
genetic predisposition to some forms of nephrolithiasis, and genes associated with cystinuria, hyperoxaluria, and X-linked nephrolithiasis have been identified.103 In many cases multiple genetic loci are involved and this reflects the many and diverse causes of hypercalciuria. A separate pathogenesis is described for struvite stones, which form as a result of chronic bacterial infection of the urinary tract, with the presence of urea-splitting bacteria being necessary for the generation of inorganic carbonate and phosphate.104
genetic predisposition to some forms of nephrolithiasis, and genes associated with cystinuria, hyperoxaluria, and X-linked nephrolithiasis have been identified.103 In many cases multiple genetic loci are involved and this reflects the many and diverse causes of hypercalciuria. A separate pathogenesis is described for struvite stones, which form as a result of chronic bacterial infection of the urinary tract, with the presence of urea-splitting bacteria being necessary for the generation of inorganic carbonate and phosphate.104
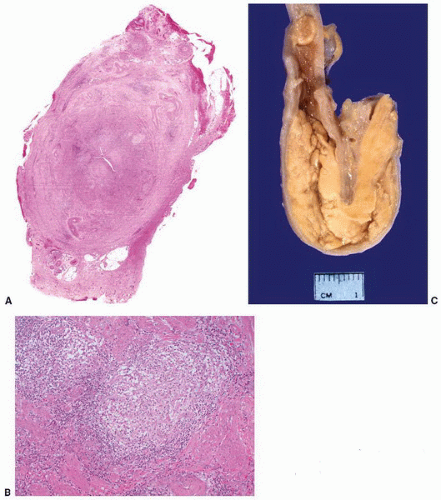 FIGURE 4-13 ▪ A: Ureteral tuberculosis with transmural thickening. B: Granulomas in ureteral tuberculosis. C: Malacoplakia involving the ureter. (Illustration courtesy of Dr. DJ Grignon.) |
Table 4-2 ▪ FREQUENCY OF TYPE OF UPPER TRACT STONE | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
The natural history of stones within the renal pelvis and ureter is dependent on stone type and the metabolic environment. In general terms calyceal stones are asymptomatic
when small, but if untreated, almost 50% will become symptomatic in 5 years.105 Larger stones within the renal pelvis may present with urinary tract infection, pain, hematuria, dysuria, and obstruction, especially at the UPJ106 (Fig. 4-14).
when small, but if untreated, almost 50% will become symptomatic in 5 years.105 Larger stones within the renal pelvis may present with urinary tract infection, pain, hematuria, dysuria, and obstruction, especially at the UPJ106 (Fig. 4-14).
Ureteral stones (Fig. 4-15) are often symptomatic, and impaction, with urinary outflow obstruction, may be a presenting feature. Stones >5 mm in diameter usually require intervention as they are unlikely to pass spontaneously,105 and complications include localized mucosal ulceration and edema, leading to scarring and stricture formation, or ureteral perforation.
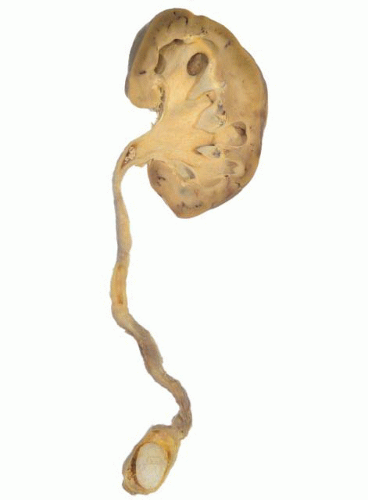 FIGURE 4-15 ▪ Ureteral stone situated superior to the vesicoureteral junction. There is dilation of the ureter and hydronephrosis. |
The pressure effect of renal stones on the mucosa of the renal pelvis is compounded by recurrent acute or chronic inflammation. This results in mucosal ulceration, reactive hyperplasia of adjacent urothelium, granulation tissue formation, and fibrosis. Chronic inflammation predisposes to reactive epithelial changes with a predominance of squamous metaplasia. Other benign mucosal changes reported from the renal pelvis in association with renal lithiasis are pyelitis follicularis, pyelitis cystica, polypoid pyelitis, xanthogranulomatous pyelitis, encrusted pyelitis, and fibroepithelial polyposis.107 Similar changes are seen in the ureter with chronic inflammation being associated with squamous metaplasia, ureteritis follicularis, ureteritis cystica, and polypoid ureteritis.107
Renal lithiasis is a recognized risk factor for renal pelvic and ureteral malignancy.108 The risk of developing malignancy is enhanced by recurrent urinary tract infections and is most commonly associated with struvite staghorn calculi. In some series the risk of malignancy increased with chronicity, while in others the risk remained stable over a 10-year follow-up.109 Urothelial carcinoma (UC) is the tumor most frequently associated with lithiasis, although squamous cell carcinoma (SCC) occurs in 20% of cases and has been found in 2% of patients with recurrent staghorn calculi.110 In addition to these tumors, verrucous carcinoma, sarcomatoid SCC, small cell (neuroendocrine) carcinoma, and adenocarcinoma have been reported.111, 112, 113
Vasculitis
Although the kidney is the most common organ to be involved in many of the forms of systemic vasculitis,114 reports of vasculitis of the renal pelvis and ureter are rare and are usually confined to single cases. Vasculitis of the urinary outflow tract often occurs as part of multisystem disease and presents as UPJ obstruction or ureteral stenosis, which may be unilateral or bilateral, with secondary hydronephrosis.
Renal pelvic or ureteral vasculitis has been reported as part of the polyarteritis group of systemic vasculitis (classic polyarteritis nodosa,115 polyarteritis nodosa associated with hepatitis B,116 and acute febrile mucocutaneous lymph node syndrome),23 as well as with Henoch-Schőnlein hypersensitivity angiitis117. In these cases ureteral stenosis was associated with typical clinical and pathologic systemic features. Renal pelvic and ureteral vasculitis has also been noted in Churg-Strauss syndrome118 and Wegener granulomatosis119 in patients with established pulmonary disease. Limited Wegener granulomatosis, involving the urogenital tract, has also been associated with ureteral vasculitis.120
Renal Pelvis and Ureter Injury
Trauma
Renal injury occurs in up to 3% of major trauma; however, direct involvement of the renal pelvis is rare.124 In cases of blunt or penetrating trauma to either the UPJ or renal pelvis, there is usually hematuria and urinary extravasation may occur. Rarely a urinoma may form and this can become secondarily infected.125,126 Ureteral trauma is most frequently iatrogenic, with gynecologic procedures predominating and in those cases requiring reconstruction, outcome is predicted by the length of ureteral injury and a history of previous radiotherapy.127 In cases of noniatrogenic ureteral injury in the United States, 81% result from gunshot wounds, while 10% are related to blunt trauma and 9% to stab wounds.128
Radiation
Ureteral stenosis has been reported in 0.3% of patients with a mean follow-up of 5.7 years following curative radiotherapy for gynecologic carcinomas.129
In the acute phase following radiotherapy, ureteral dilation occurs in two-thirds of cases, and this usually resolves over 6 months.130 In experimental animal models asymptomatic fibrotic ureteral stenosis was observed in dogs following doses of 20 Gy, and this has been associated with increased levels of TGF-β1.131
High-dose radiation is associated with capillary thrombosis, and in early lesions epithelial atypia and mucosal ulceration are seen. Chronic effects consist of cytoplasmic vacuolation of epithelial cells, loss of pleating with associated telangiectasia of ureteral mucosa, submucosal chronic active inflammation and fibrosis (Fig. 4-16), ureteritis cystica, and squamous metaplasia.129,132,133 In experimental animal models these changes have been shown to increase in severity for the first 12 months following radiotherapy. In these studies the resulting stenosis was found to be functional as secondary hydronephrosis developed in the presence of a patent ureter.132 Ureteral UC has been reported as a secondary effect of radiation,134 and renal pelvis SCC has also been noted.135
Renal Pelvis Hemorrhage
Hemorrhage into the renal pelvis may be associated with subepithelial hematoma (Antopol-Goldman lesion).136 Often misdiagnosed as renal pelvic neoplasia, 25 cases of subepithelial hematoma have been reported. There is a female predominance with the ages of patients ranging from 24 to 84 years.136, 137, 138 In several cases the cause of the hematoma could not be determined, while in others it was shown to be secondary to trauma, hypertension, analgesic abuse, or coagulopathy. In five cases subepithelial hematomas were associated with congenital renal abnormalities.136, 137, 138 Histologic findings are nonspecific and consist of subepithelial hemorrhage, which may be associated with peripelvic hemorrhage, renal cortical infarction, or hydronephrosis.138 The majority of cases have been treated by nephrectomy or partial nephrectomy; however, once the diagnosis is established, conservative therapy is advocated.138
Retroperitoneal Fibrosis (Sclerosing Retroperitoneal Fibrosis)
Retroperitoneal fibrosis is typically a diffuse fibrous process that involves the aorta, vena cava, and structures of the retroperitoneum. It may be primary or idiopathic or in approximately 25% of cases is secondary to autoimmune diseases, malignancy, trauma and surgery, chronic infection, drugs (methysergide, pergolide, ergotamine, methyldopa, hydralazine, and beta-blockers), and radiation.141 There is a prevalence of 1 to 2 cases per 200,000 with a male predominance, and although pediatric cases are well recognized,142 the majority of patients are 50 to 60 years of age at diagnosis.143
The pathogenesis of retroperitoneal fibrosis is uncertain, and the association of secondary forms of the disease with hyperimmune states suggests an autoimmune etiology. There is also a reported association with asbestosis, autoimmune thyroid disease including Riedel thyroiditis, Wegener granulomatosis, mediastinal fibrosis, polyarteritis nodosa, systemic lupus erythematosus, sclerosing cholangitis and primary biliary cirrhosis, UC, and sclerosing lymphoma.144
Clinical features are nonspecific and relate to the structure encased in fibrous tissue. Often there is ureteral involvement, and in neglected cases renal failure is a common complication. Laboratory results are similarly nonspecific
with elevated C-reactive protein and erythrocyte sedimentation rate, as well as positive antinuclear antibodies being the most frequent findings.
with elevated C-reactive protein and erythrocyte sedimentation rate, as well as positive antinuclear antibodies being the most frequent findings.
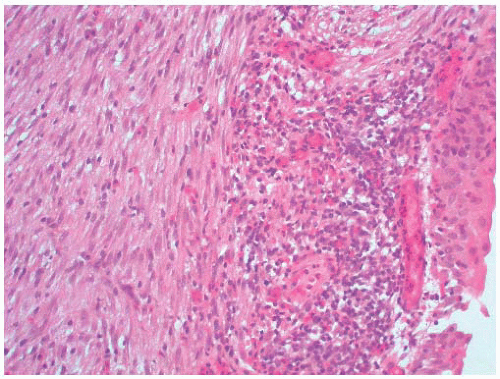
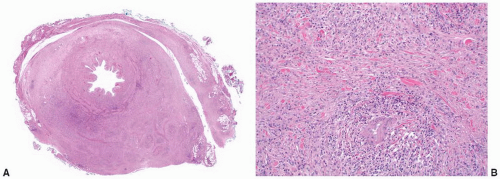
Macroscopically, retroperitoneal fibrosis consists of white fibrous plaques that encase retroperitoneal structures. The fibrosis is usually diffuse; however, in occasional cases the fibrosis is confined to single organs, and localized ureteral (Fig. 4-17A) and perirenal retroperitoneal fibrosis has been reported.145 On microscopic examination (Fig. 4-17B) the lesions consist of sheets of fibroblasts showing variable cellularity. There is frequently an associated inflammatory infiltrate consisting predominantly of lymphocytes and plasma cells. In older lesions the inflammatory cell infiltrate is less prominent, and there is hyalinization, occasionally with dystrophic calcification.141 Vascular changes resembling vasculitis, with intramural inflammatory cells and fibrinoid necrosis, are often present.
In the majority of cases, there is both a symptomatic and clinical response to steroids with a resulting decrease in the volume of fibrous tissue.141 Stenting of ureters is frequently necessary, and in cases refractile to steroids, surgical intervention, consisting of ureteral replacement and autotransplantation of the kidney may be required. Often the disease relapses and long-term follow-up is necessary.143 In cases of secondary retroperitoneal fibrosis, treatment of the primary disease process may lead to a reduction in the volume of fibrous tissue.
OTHER LESIONS
Reactive Atypia Due to Chemotherapeutic Agents
Topical chemotherapeutic agents such as triethylenethiophosphoramide (thiotepa) and mitomycin C are used to treat superficial urothelial cancer of the upper tract and may produce urothelial atypia.146,147 Reactive urothelial atypia may also be caused by chemotherapeutic agents given systemically to treat neoplastic and nonneoplastic disorders. Among these, cyclophosphamide is known to have significant effects on urothelium and can reactivate polyoma virus infection, which morphologically can also mimic carcinoma. busulfan, adriamycin, epirubicin, ethoglucid, cisplatin, and mitoxantrone are other agents known to cause urothelial changes.147
Thiotepa and mitomycin C cause a pronounced necroinflammatory process and mucosal denudation accompanied by enlargement, vacuolization, and multinucleation of urothelial cells. These changes can last for several weeks after treatment.147 Cyclophosphamide produces variable cellular and nuclear enlargement, with binucleation and multinucleation. In these cases, usually there are large, bizarre nuclei resembling those seen after radiotherapy. Nuclei are often eccentric, irregular in outline, and hyperchromatic, with coarse chromatin mimicking carcinoma cells. However, unlike carcinoma, there is loss of chromatin texture with nuclear pyknosis. Cyclophosphamide may also cause hemorrhagic pyelitis and ureteritis, in addition to cystitis. The histologic changes include vascular ectasia with severe edema and hemorrhage of the lamina propria, usually associated with necrosis of the epithelial lining, and mucosal ulceration.148
Reactive Atypia Due to Instrumentation, Stents, and Strictures
Biopsies from patients with stents or strictures and after instrumentation can have atypical changes mimicking urothelial CIS. In contrast to urothelial CIS, reactive atypia displays cellular and nuclear enlargement with prominent nucleoli and mitotic activity in the absence of significant pleomorphism and chromatin abnormalities. Immunohistochemistry (CK20, P53, and CD44) may be helpful however, correlation with morphology is critical due to overlap in immunoprofile in reactive versus neoplastic conditions.
Endometriosis and Müllerianosis
Endometriosis, the benign proliferation of ectopic endometrial mucosa, involves the urinary tract in 2% of reported cases.149 In most of these instances, endometriosis is confined to the urinary bladder; however, in 16% of cases one or both ureters are also involved.150 In most cases of urinary tract endometriosis, symptoms of dysuria, frequency, recurrent urinary tract infection, and/or renal angle pain are reported, although endometriosis confined to the ureter is asymptomatic in 60% of patients.151
In endometriosis diffusely spread within the pelvis and in the urinary tract, lesions are more commonly found on the left side, and it has been speculated that this is a reflection of the direction of flow of peritoneal fluid that carries regurgitated endometrial tissue to ectopic sites.149
In 65% of cases involving the ureters, endometriosis is superficial within the overlying peritoneal tissues, and in a further 30% of cases, the endometriotic deposits lie adjacent to the ureter. Intramural endometriosis is present in only 5% of lesions, and these are usually associated with ureteral stenosis.150,152
The histologic features of ureteral endometriosis are similar to those seen in other organs. Recognizable endometrial epithelium and stroma may be present, or there may be recent hemorrhage and/or aggregates of hemosiderin and hemosiderin-laden macrophages (Fig. 4-18). In the latter instance CD10 immunohistochemistry is often useful for detecting small foci of residual endometrial stroma. Rarely, Müllerianosis with endocervicosis or endosalpingiosis can be seen as ureteric lesions. In some cases there is associated endometriosis.153
Rarely endometrioid adenocarcinoma may arise in ureteral endometriosis, and in one reported case there was no evidence of residual endometriosis in the genital tract or elsewhere in the urinary tract.154
Management of endometriosis of the ureter is dependent on the site of the lesion. Peritoneal deposits are amenable to laparoscopic excision, while intramural endometriosis may require ureteral resection and anastomosis.153,155
Amyloidosis
Primary localized amyloidosis is rarely seen in the urinary tract with <50 reported cases involving the renal pelvis and ureter.156, 157, 158 There is a male predominance, and patients are usually >50 years of age, with pyrexia, hematuria, and flank pain being common presenting features.156 On imaging, ureteral stricture and hydronephrosis are frequent findings. This may be unilateral or bilateral and in most cases mimics invasive UC.157 Although urinary cytology may provide evidence of the correct diagnosis,157 most cases are diagnosed following surgery.
Histologically, amyloid deposits are interstitial and most commonly involve the lamina propria and muscularis. The amyloid is usually of the AL type, as demonstrated by congophilia with resistance to permanganate treatment, and on immunohistochemistry consists of lambda light chains. Rarely osseous metaplasia may occur.159 Management usually involves surgical resection of the involved segment.
HYPERPLASIA AND METAPLASIA
Pyeloureteritis Cystica and Glandularis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree