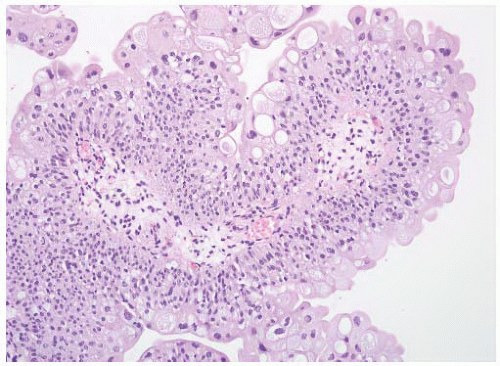
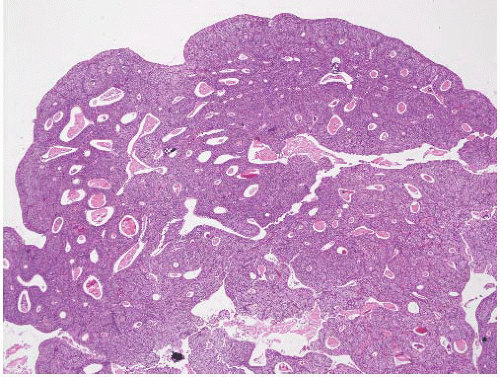
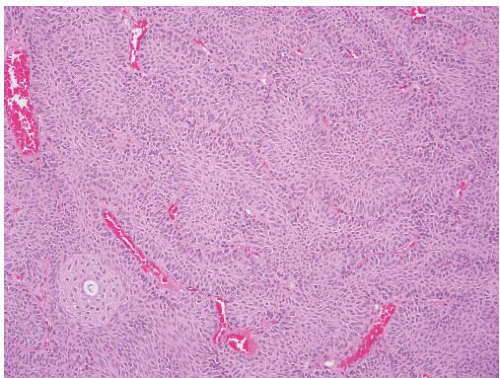
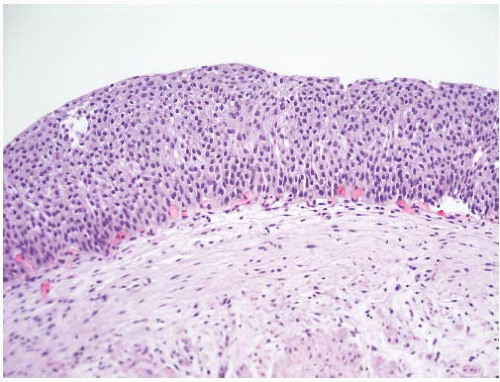
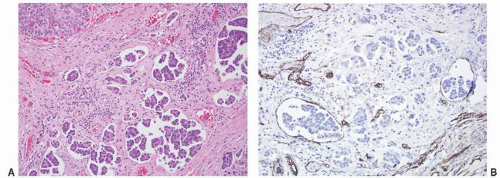
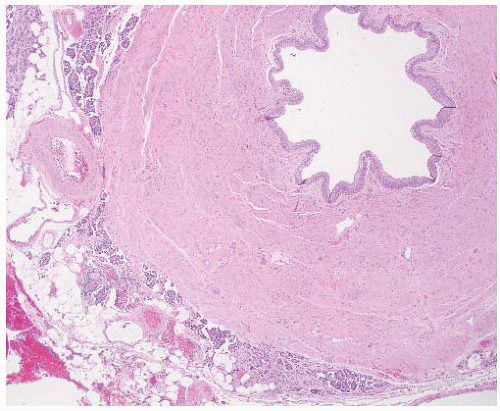
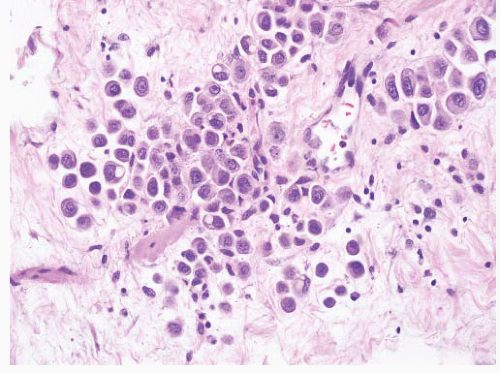
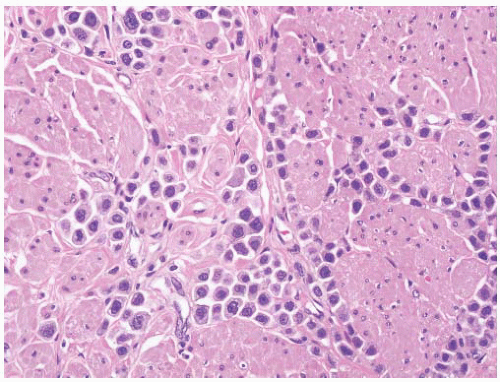
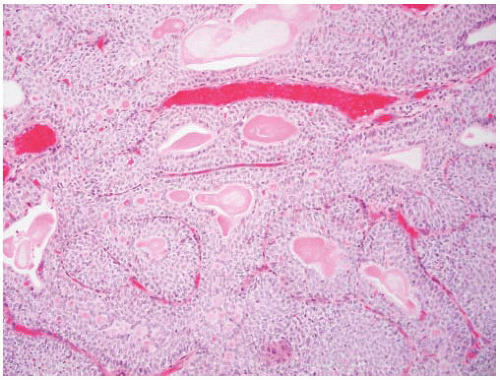
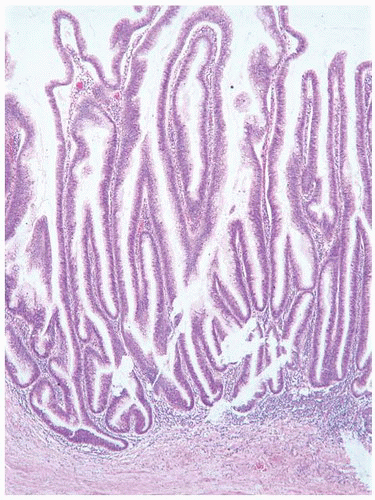
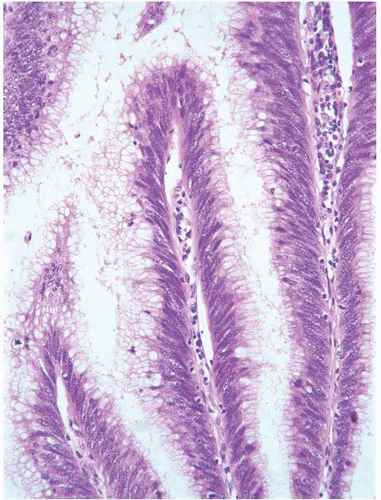
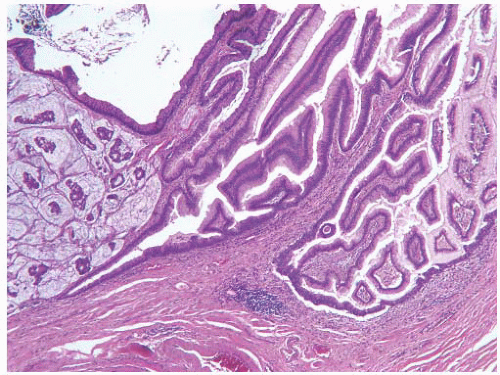
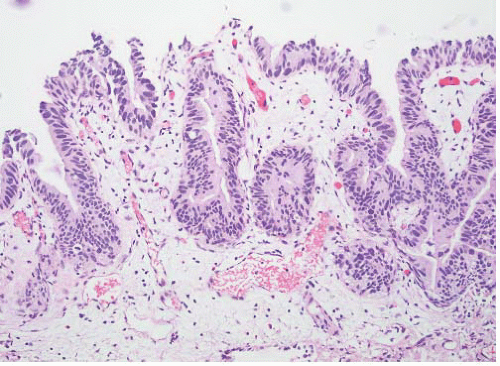
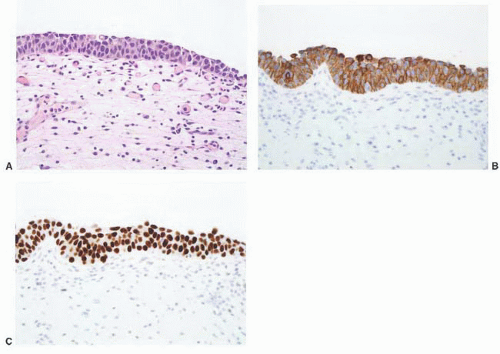
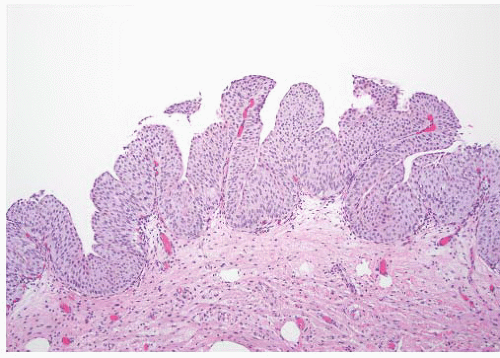
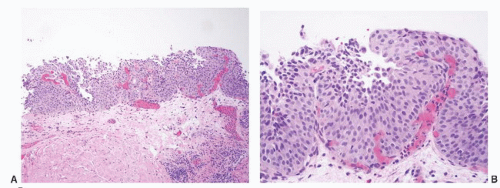
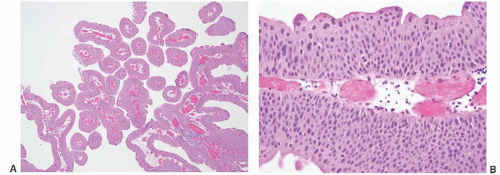
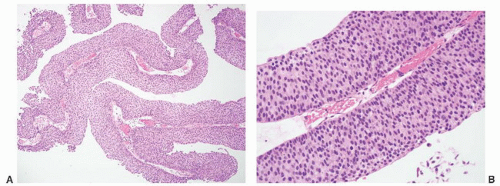
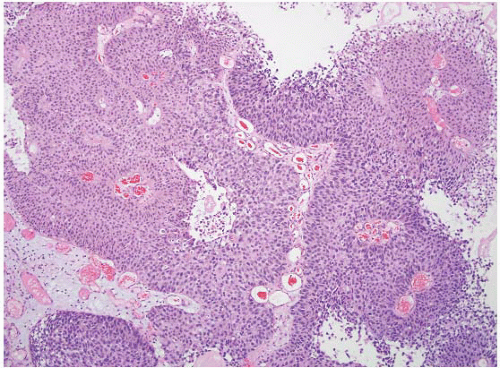
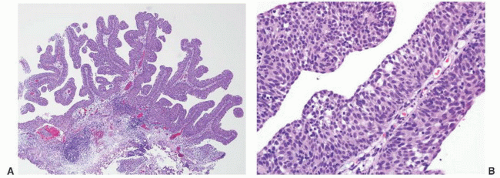
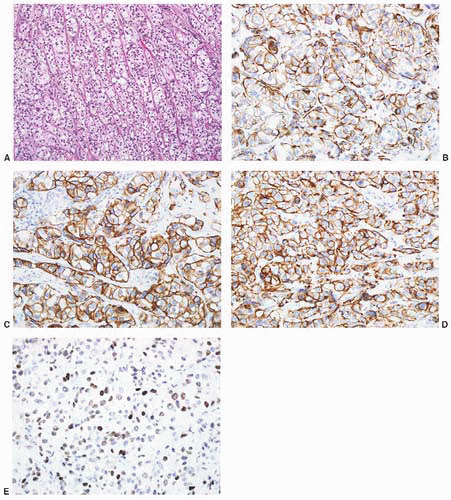
CLASSIFICATION OF UROTHELIAL TUMORS
The last decade has seen a tremendous upheaval in this very important area that only recently seems to have settled down to a level of general concurrence.
1 For over two decades, the World Health Organization (WHO) classification and grading of urothelial neoplasms
2 dominated although several variations and different schemes were published. In the early 1990s, several factors emerged that resulted in the need to reevaluate this approach. First, the controversy of calling grade 1 papillary tumors “carcinoma” arose with several groups led by Dr. William Murphy calling all tumors in the low-grade end as papilloma.
3,
4 Second, the use of intravesical therapy as a standard practice in the treatment of highrisk noninvasive papillary tumors demanded that high-risk tumors be clearly identified. Third, the WHO (1973) system was criticized for the imprecision of the published criteria (
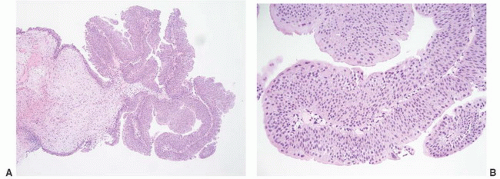
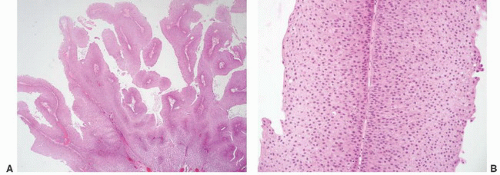
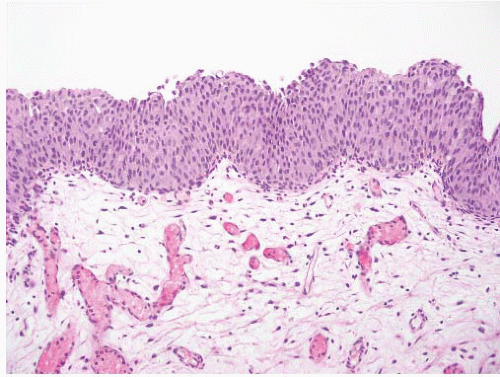
Table 6-1), leading many pathologists to essentially use this three-grade system to create five grade groups (1, 1-2, 2, 2-3, and 3), one result being that only a small percentage of cases were placed in the grade 3 group. For example, in a review of three clinical studies only 25 of 280 (8.9%) newly diagnosed noninvasive papillary tumors (pTa) were called grade 3.
5 The effect of the latter was confusion as to how to treat grade 2 Ta tumors, a category that included many high-risk patients who could benefit from intravesical therapy as well as many patients with low-risk disease for whom intravesical therapy may not be appropriate or necessary. Multiple studies have demonstrated that this group includes high-risk patients with progression to invasive carcinoma reported in up to 20% of patients and cancer-specific death in 13% to 20%.
6,
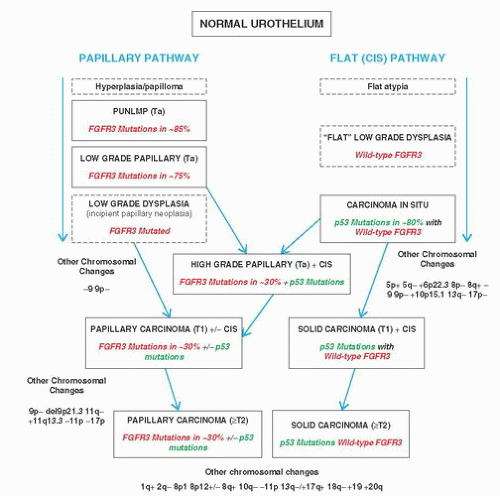
7Recognizing the many emerging issues, in 1997 Dr. F. K. Mostofi organized a meeting of a small group of urologic pathologists, urologists, and urologic oncologists to address these concerns. This was followed by a much larger consensus conference that was held under the auspices of the International Society of Urologic Pathology (ISUP) in March of 1998. The results of this consensus were adopted by the WHO and the results published in 1998 as the WHO/ISUP consensus classification (
Table 6-2).
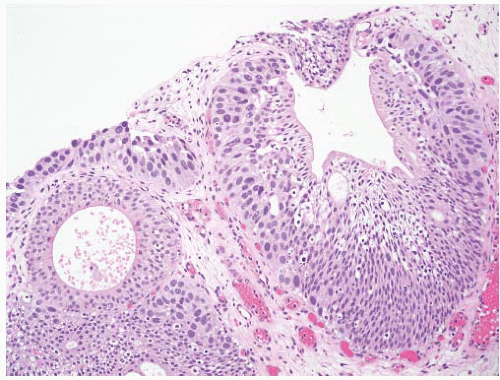
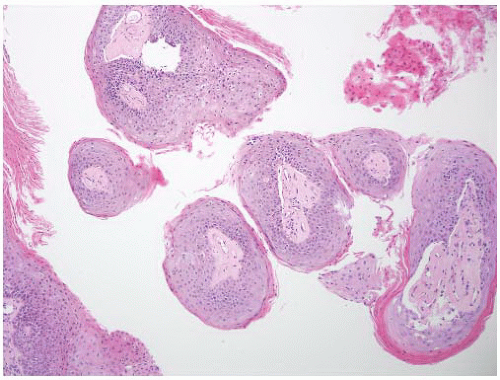
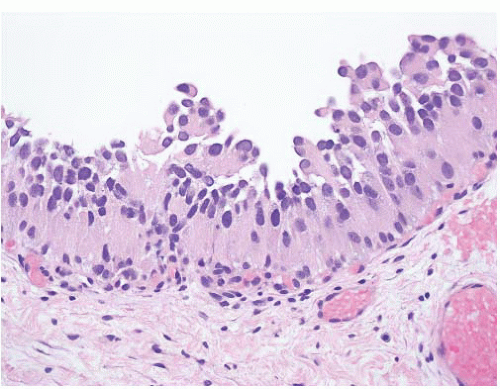
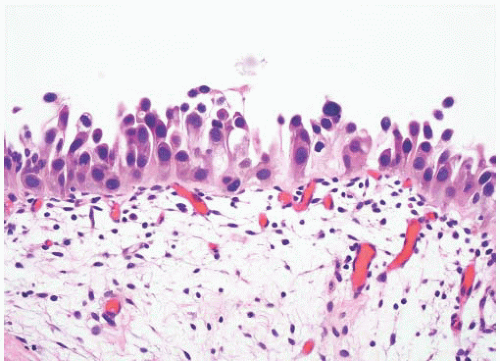
8 Most controversial was the adoption of the term papillary urothelial neoplasm of low malignant potential. This represented a compromise term where the papilloma and carcinoma advocates could be comfortable and allowed that controversy to be brought to resolution. Most important was the adoption of the grading system that had been described by Malmström et al.
9 The value of the latter was viewed as twofold; first, the morphologic criteria for applying the scheme were well defined and, second, it appeared to place the majority of patients with high-risk disease into the high-grade category.
The publication of the 1999 WHO blue book the following year introduced a variation on this system with the splitting of the low- and high-grade categories of the 1998 WHO/ISUP classification into three groups (grades 1, 2, and 3) while retaining the papillary urothelial neoplasm of low malignant potential category.
10 This reignited the controversy with some experts urging a return to the 1973 WHO grading system.
11 Others criticized the 1998 WHO/ISUP system as simply representing a renaming of the 1973 WHO,
12 a clearly incorrect interpretation.
13 At a subsequent meeting in Ancona, Italy (2001), a modified version of the 1973 WHO was proposed.
13This issue became the primary focus of discussion at the WHO meetings prior to the release of the 2004 WHO classification. Following extensive debate and discussion, it was agreed overwhelmingly to essentially reproduce the 1998 WHO/ISUP classification as the 2004 WHO recommended classification scheme (
Table 6-2).
14 The authors of the Fourth Series Armed Forces Institute of Pathology fascicle covering bladder neoplasia also followed this system.
15 With this consistent approach adopted by arguably the two most influential references for tumor classification and grading, work can continue on evaluating the biologic and clinical relevance and value of this system.
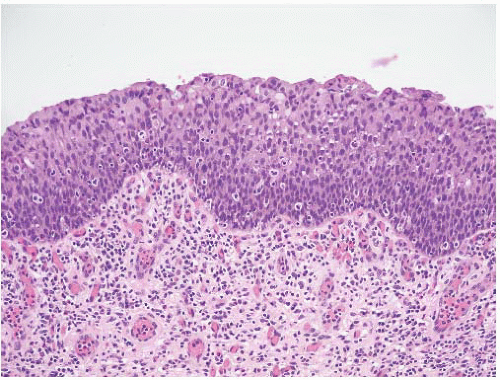
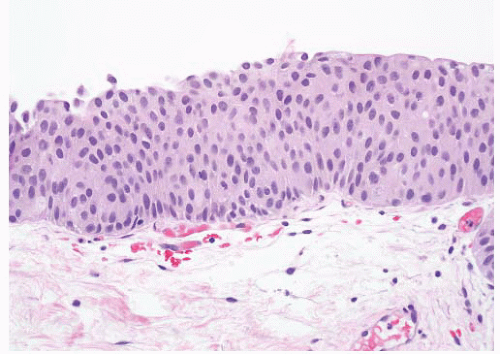
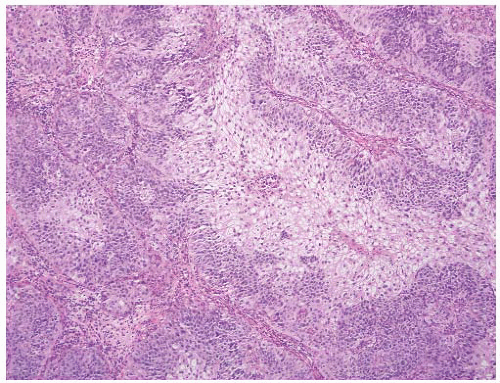
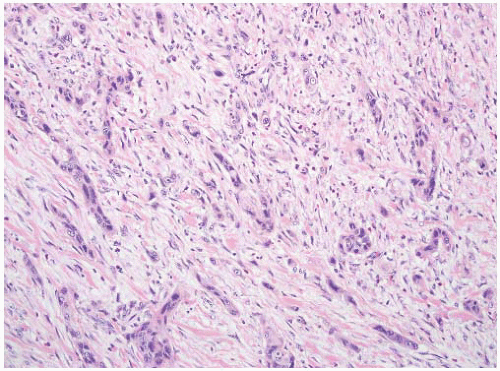
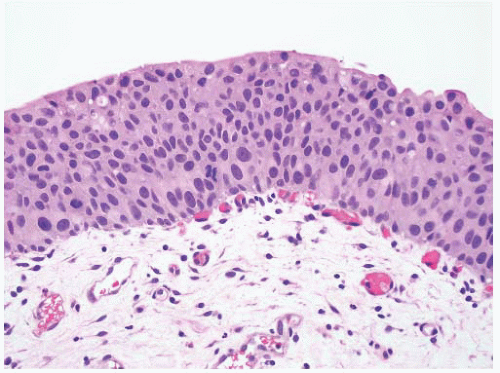
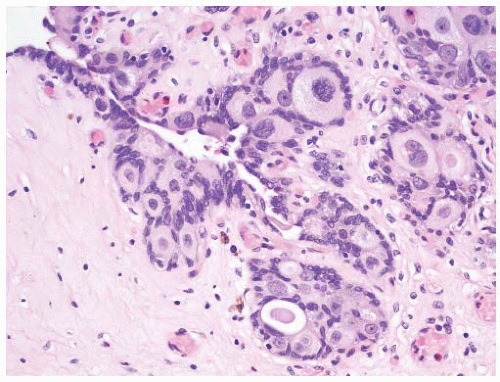
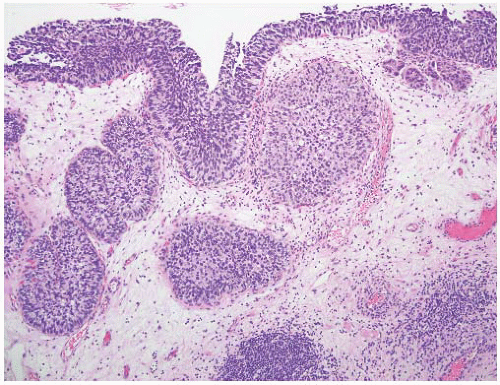
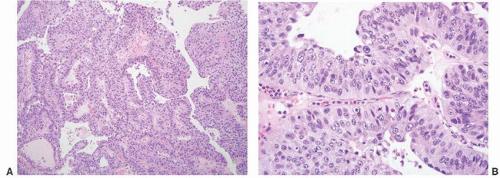
16The success of the 2004 WHO/ISUP classification in addressing the key issues outlined in the first paragraph has been stressed, in particular the reclassification of high-risk
grade 2 tumors (WHO 1973) into the high-grade papillary carcinoma category.
13,
17,
18 For example, in the study by Yin and Leong,
18 13 of 46 WHO (1973) grade 2 tumors (28%) were placed in the WHO (2004) high-grade category resulting in 23% of all cases being considered high-grade in WHO 2004 compared to only 4% being called grade 3 in the 1973 WHO system. Similarly, Samaratunga et al.
17 reviewed 134 papillary tumors of which 6 (4%) had been reported as grade 3 (WHO, 1973); on review they considered 29 (22%) to be high grade by WHO/ISUP 1998.
This approach has been embraced by many urologic oncologists with interest in bladder cancer. It has important application in the contemporary treatment of Ta tumors. The reclassification of high-risk grade 2 tumors (WHO 1973) into the high-grade category (WHO 2004) has resulted in a large and better-defined group of patients with low-risk Ta tumors. In a series of 215 patients with low-grade (papilloma, papillary urothelial neoplasm of low malignant potential and lowgrade papillary carcinoma) Ta tumors from the Memorial Sloan Kettering Cancer Center treated by transurethral resection only, progression to high-grade Ta or invasive carcinoma occurred in only 3% and 5% of patients, respectively, with a median follow-up of 8 years.
19 A prospective study lent further support to these findings.
20 This experience has led to the suggestion that patients with low-grade Ta tumors can be followed less frequently.
21 Similarly, Nieder and Soloway
22 recommended treating patients with papilloma and papillary urothelial neoplasm of low malignant potential by transurethral resection alone, low-grade Ta tumors by transurethral resection with a single dose of mitomycin C, and highgrade Ta tumors by transurethral resection with mitomycin C and bacillus Calmette-Guérin (BCG) immunotherapy. In December 2007, the American Urological Association (AUA) reinforced the current approach in its guidelines for the treatment of non-muscle-invasive bladder cancers by separating noninvasive papillary tumors into two groups— low grade and high grade
23—with different treatment recommendations for each group. The use of this system has also been advocated by the European Urology Association.
24 The College of American Pathologists (CAP) utilizes WHO 2004 in its recommendations for the reporting of urothelial tumors.
25,
26 Support for this system was further emphasized in the International Consultation on Bladder Cancer.
27