Other Tumors of the Prostate Gland and Tumors of the Seminal Vesicle
JONATHAN I. EPSTEIN
FADI BRIMO
OTHER TUMORS OF THE PROSTATE GLAND
Adenosquamous and Squamous Cell Carcinoma
Clinical Features
These are very rare tumors with approximately 55 cases of pure squamous cell and 30 cases of adenosquamous cell carcinoma reported. Risk factors include prior radiation or hormonal therapy, previous history of prostate cancer, and rarely genitourinary schistosomiasis. However, de novo cases without prior history of cancer have been reported. The mean age of diagnosis is 64 years. Patients usually present with obstructive symptoms and sometimes hematuria. While increased prostate-specific antigen (PSA) and acid phosphatase levels is seen more commonly with adenosquamous in comparison to pure squamous cell carcinomas, normal levels are not uncommonly present even with advanced disease.1
Pathology
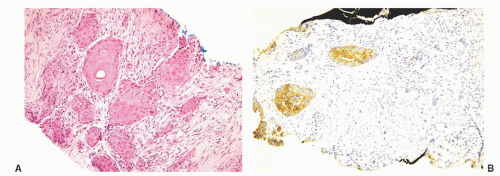
Pure squamous cell carcinoma of the prostate is indistinguishable from squamous carcinoma at other sites. Adenosquamous carcinoma may show an intimate mixture of prostatic acinar adenocarcinoma and squamous cell carcinoma (Fig. 10-1). Alternatively, the two components may be more anatomically distinct with squamous cell carcinoma seen in one area of the specimen and acinar adenocarcinoma in another. The extent of adenocarcinoma varies from 5% to 95% and tends usually to be higher grade tumors with a Gleason score of 7 or higher. The degree of differentiation within the squamous component can also range from well to more poorly differentiated.1
Differential Diagnosis
Primary prostatic squamous cell carcinomas must be distinguished on clinical grounds from secondary involvement of the gland by bladder, urethral, or anal pure squamous cell carcinomas. Histologic distinction between those entities is extremely difficult and generally requires clinicopathologic correlation. A more common scenario is secondary invasion by a urothelial carcinoma with squamous differentiation, and in that regard, features that are most consistent with secondary urothelial carcinoma with squamous differentiation rather than a prostatic primary tumor include (1) the presence of a component of usual-type invasive urothelial carcinoma, (2) associated glandular (nonprostatic-type) differentiation, (3) associated urothelial carcinoma in situ or urothelial papillary tumor in the bladder lining. Also, squamous cell carcinoma of the prostate must be differentiated from squamous metaplasia adjacent to a prostatic infarct. In transurethral resections and enucleations, the localized lobular nature of infarcts with the organization from central necrosis and reactive urothelial/squamous islands at the periphery is diagnostic. On needle biopsy, the overall architecture cannot be as easily appreciated. Although the squamous/urothelial nests in an infarct usually lack cytologic atypia, prominent atypia and increased mitotic activity may occasionally be seen. The recognition of adjacent infarcted tissue or the presence of stromal hemorrhage and hemosiderin deposition in the areas adjacent to the infarct is helpful in arriving at the correct diagnosis.
Lastly, squamous changes in benign prostatic tissue following hormonal therapy can be distinguished from carcinoma due to the bland cytological characteristics and the absence of invasive features.
At the immunohistochemical level, while cells in the squamous cell carcinoma component are usually totally negative for prostatic markers (prostate-specific acid phosphatase [PSAP], PSA, prostate-specific membrane antigen [PSMA], prostein) and positive for p63, HMWK, CK5/6, CK14, the glandular component of the adenosquamous cell carcinoma show the opposite pattern of staining.1
Prognosis and Treatment
Primary prostatic squamous cell carcinomas have a poor prognosis, with poor response to hormonal therapy, radiation therapy, and chemotherapy. Radical prostatectomy constitutes the only chance of cure, although the majority of cases present with advanced disease.2 Metastases occur in a third of cases, most commonly to bone and lung. Squamous cell carcinomas develop osteolytic rather than osteoblastic metastases and do not respond to antiandrogen therapy.
Sarcomatoid Carcinoma
Introduction
Some authors restrict the term “carcinosarcoma” for tumors with carcinoma and specific mesenchymal differentiation (i.e., cartilage or osteoid), and designate tumors with only an associated nonspecific malignant spindle cell pattern as “sarcomatoid carcinoma.” Given the similar prognosis between these two morphologies and the finding of epithelial differentiation by immunohistochemistry or electron microscopy in the spindle component of some “carcinosarcomas,” it is preferable to consider these lesions as one entity.3
Clinical Features
Sarcomatoid carcinomas of the prostate are rare with only approximately 100 cases and few reported series.4, 5, 6, 7 Prior history of prostatic adenocarcinoma is present in about half of cases in whom the sarcomatous component develops at a mean of 7 years later (range = 6 months to 16 years). Radiotherapy and/or hormonal therapy are thought to play a role in the development of sarcomatoid carcinoma as some patients have received these treatment modalities prior to the growth of the sarcomatoid component. This being said, sarcomatoid carcinoma may also arise de novo without prior history of adenocarcinoma or previous treatment. Mean age at diagnosis is 70 years, and the majority of patients present with obstructive urinary symptoms or metastatic disease rather than by being detected by needle biopsy following an increase in serum PSA levels or abnormal digital rectal examination. PSA serum levels may be normal or elevated.
Pathology
Gross findings are often not appreciable as the specimens are typically needle biopsies or transurethral resections. However, tumors removed by radical prostatectomy may resemble sarcomas and present as large white to yellow-tan tumors with necrosis and hemorrhage and fish flesh appearance.
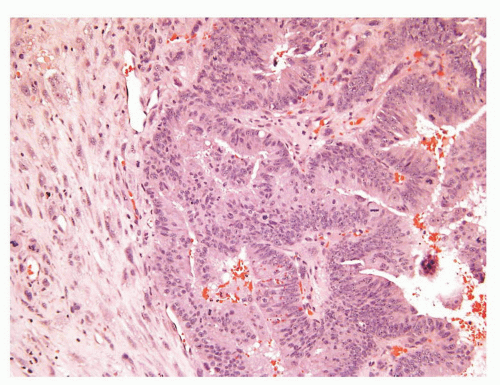
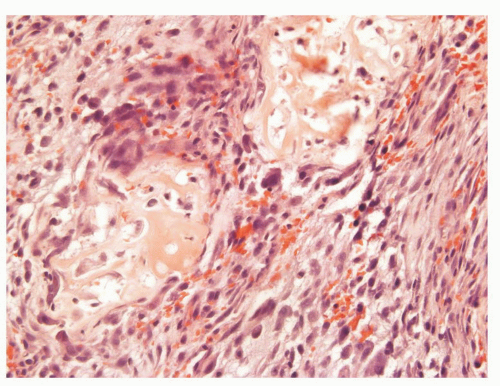
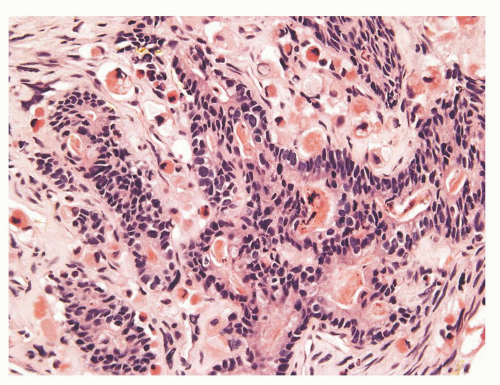
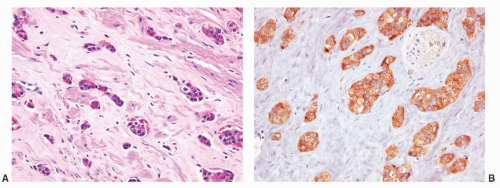
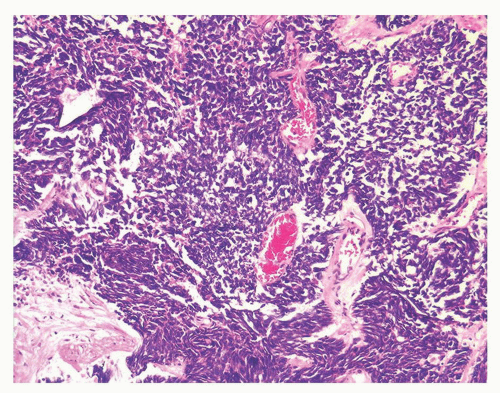
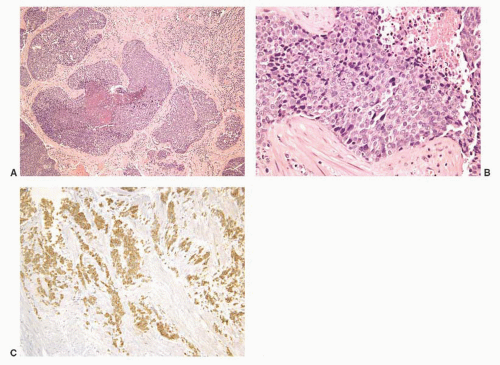
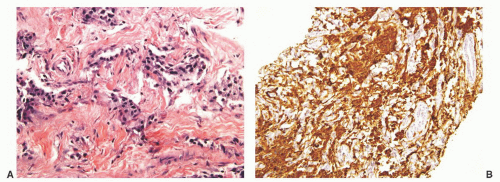
The overall histology shows one of the following three patterns: (1) intimate admixture of carcinoma and sarcomatoid carcinoma with nonspecific spindle cell morphology, this being the most common type, (2) carcinoma admixed with sarcomatous component showing heterologous differentiation, and (3) pure spindle cell tumor. The epithelial component of carcinosarcoma is either moderately or poorly differentiated adenocarcinoma, usually acinar but occasionally ductal, small cell, adenosquamous, or basal cell carcinoma. The acinar adenocarcinoma component is often high-grade (Gleason score 8 to 10). The spindle cell component commonly is undifferentiated, or shows chondroid or osteoid differentiation (Figs. 10-2, 10-3 and 10-4). Less commonly, the spindle component differentiates toward rhabdomyosarcoma, leiomyosarcoma, or angiosarcoma (Fig. 10-5) and shows variable immunoreactivity for vimentin, desmin, actin, smooth muscle actin (SMA), and S100 protein. Typically, the spindle component is negative for prostatic markers (PSA, PSAP, PSMA, p501s) but may be focally positive. Low molecular weight cytokeratin is virtually always positive although its reaction with other keratin antibodies (PAN-CK, HMWK, CK5/6) is seen in only half of the cases. Vimentin is positive.7 Electron microscopy of the sarcomatoid areas may reveal desmosomes.
Differential Diagnosis
The main differential diagnoses are the following:
Primary sarcoma of the prostate: This occurs in sarcomatoid carcinomas in which the epithelial component is focal or absent. The most common prostatic sarcomas are stromal sarcomas and leiomyosarcomas. In general, extensive sampling and demonstration of an associated epithelial component is consistent with sarcomatoid carcinoma rather than sarcoma. It should be mentioned, however, that stromal sarcoma can have an associated epithelial component in the subtype called “phyllodes-like”; however, the epithelial component is benign in these tumors. Also, stromal sarcomas are keratin-negative and express CD34 and progesterone receptor (PR). Leiomyosarcomas may, however, occasionally show focal keratin positivity, although diffuse staining is not seen. As a general rule, keratin positivity within the spindle cell component of a lesion, which lacks the typical morphology of one of the sarcomas that can express keratin, is diagnostic of sarcomatoid carcinoma. For example, the sarcomatous component in sarcomatoid carcinoma typically consists of a haphazard array of malignant spindle cells, as opposed to the intersecting parallel bundles of malignant smooth muscle cells seen in leiomyosarcoma. High molecular weight cytokeratin and CK5/6 are two of the better keratins to utilize in this regard.
Inflammatory myofibroblastic tumor (IMT): Features that are distinctive from sarcomatoid carcinoma include the tissue culture-like appearance, the typical granulation tissue-type vascularity and associated inflammatory cells, the lack of nuclear pleomorphism, and hyperchromasia. A further pitfall is that IMTs are positive
for keratin and may express SMA. Approximately two-thirds of IMTs are positive for ALK1, which is diagnostic of IMT. One should, however, be aware of several atypical morphologic features that can be seen in IMT in order not to overdiagnose them as sarcoma or sarcomatoid carcinoma. Those include hypercellularity, infiltrative pattern of growth, necrosis, and increased mitotic activity, although atypical mitotic figures are not seen.
Gastrointestinal stromal tumor (GIST): Rarely those tumors secondarily involve the prostate and are composed of uniform spindle cells with palisading and perinuclear halos, without the pleomorphism of sarcomatoid carcinoma. GISTs are keratin negative and positive for CD117, DOG-1, and CD34.
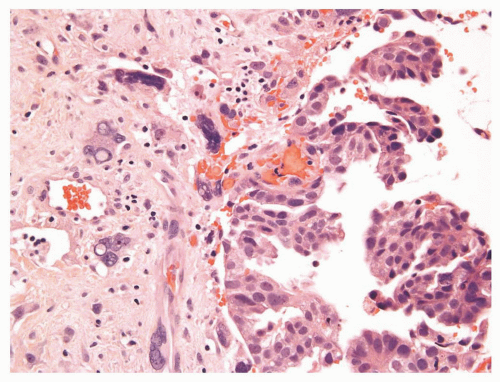 FIGURE 10-3 ▪ Sarcomatoid carcinoma with acinar adenocarcinoma (right) and undifferentiated sarcomatoid component consisting of malignant giant cells (left). |
Prognosis and Treatment
Sarcomatoid carcinomas have an aggressive clinical course and a dismal prognosis with an actuarial risk of death at 1 year up to 20% and 14% survival rates at 7 years postdiagnosis. Metastatic lesions may be purely glandular, mixed carcinosarcomatous, or purely sarcomatous. The treatment is the same as for usual acinar adenocarcinoma of the prostate. However, current therapies, including multimodal approaches, remain ineffective and not standard.
Neuroendocrine Differentiation in Acinar Prostatic Adenocarcinoma
Even in ordinary adenocarcinomas of the prostate without light microscopic evidence of neuroendocrine differentiation, between 30% and 100% show neuroendocrine differentiation when evaluated with immunohistochemistry for multiple neuroendocrine markers. These cells may react with antibodies to chromogranin, serotonin, adrenocorticotropic hormone (ACTH), calcitonin, human chorionic gonadotropin, neuron-specific enolase, somatostatin, leuenkephalin, or beta-endorphin.8 The vast majority of these cases have no clinical evidence of ectopic hormonal secretion.
Androgen-deprivation therapy is associated with an increased number of neuroendocrine cells.9 It is suggested that growth-promoting neuropeptides elaborated by the neuroendocrine cells sustain the growth of the nonneuroendocrine carcinoma cells in an androgen-deprived environment.10, 11, 12 Furthermore, androgen depletion may lead to transdifferentiation of carcinoma cells into androgen receptor negative, apoptosis-resistant (bcl-2 positive) neuroendocrine cells.13,14
It is controversial whether neuroendocrine differentiation in typical adenocarcinomas worsens prognosis, with some studies suggesting a correlation8,15,16 yet most others showing no effect of neuroendocrine differentiation on outcome.17,18 Currently, in accordance with the College of American Pathologists Consensus Statement 1999, neuroendocrine differentiation remains a category III prognostic factor together with other factors not sufficiently studied to demonstrate their prognostic value.19
Adenocarcinoma with Paneth Cell-Like Neuroendocrine Differentiation
In histologically typical adenocarcinomas of the prostate and occasionally in high-grade prostatic intraepithelial neoplasia (HGPIN), basally located deeply eosinophilic fine cytoplasmic granules can be identified that are chromogranin positive and contain neurosecretory granules by electron microscopy (Figs. 10-6 and 10-7). The term Paneth cell-like has been used to describe these eosinophilic neuroendocrine cells.20 Paneth cell-like neuroendocrine differentiation in prostatic adenocarcinoma can be seen as either patchy isolated cells or diffusely involving glands or nests.21,22 Despite the cells’ bland histologic appearance, strictly applying the Gleason grading system one would assign a Gleason pattern 5 to Paneth cell-like foci with no glandular formation (Fig. 10-8). It has been shown that such grade assignment does not reflect the clinical behavior in these cases. It is therefore recommended that Gleason grading in such tumors be assigned only to the areas of conventional adenocarcinoma. If the entire tumor consists of sheets, cords, or nests with Paneth cell-like neuroendocrine differentiation, then a Gleason score should not be assigned and a comment to the generally favorable prognosis of these lesions could be provided.21
Carcinoid Tumor
The majority of lesions reported as “carcinoid tumors” of the prostate are merely prostatic adenocarcinomas with neuroendocrine differentiation. Almost all such cases have been positive with antibodies for PSA and PSAP and have clinically behaved like ordinary prostate carcinomas. True carcinoid tumors of the prostate with negative immunoreactivity for PSA and PSAP and otherwise typical carcinoid tumor morphology and immunoprofile are extremely rare (Fig. 10-9).23, 24, 25, 26, 27
Small Cell Carcinoma
Clinical Features
Although the prostate is one of the most common sites of extrapulmonary small cell carcinoma, prostatic small cell carcinoma is rare, comprising 0.5% to 2% of all prostatic carcinomas. In the largest series of prostatic small cell carcinoma, there was a prior diagnosis of usual adenocarcinoma of the prostate in 42% of cases.28 The median time interval between the diagnosis of prostate cancer and small cell carcinoma in this study was 25 months with some patients having had a prior history of hormonal therapy. The mean age at presentation is 69 years, and typically patients present with a rapid onset of urinary tract obstruction. Although most cases lack clinically significant hormonal production, some patients present with paraneoplastic symptoms such as ACTH or ADH overproduction or Eaton-Lambert syndrome. Serum PSA levels are variable, and typically are normal. A classic scenario is that patients have very elevated serum PSA levels with metastatic disease and then the PSA levels fall indicating the onset of a small cell component.
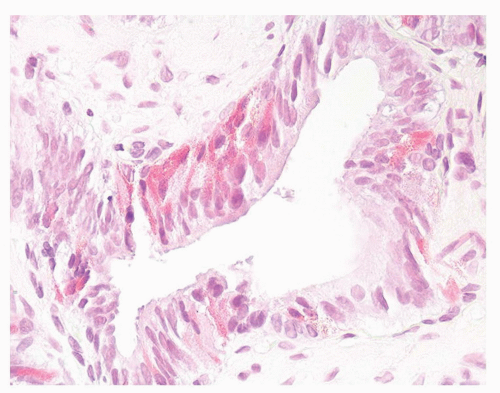 FIGURE 10-6 ▪ High-grade prostatic intraepithelial neoplasia with Paneth cell-like neuroendocrine change. |
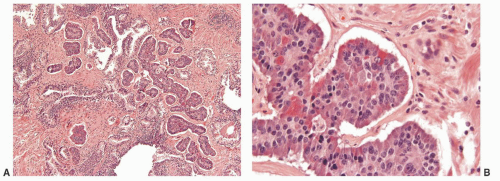 FIGURE 10-9 ▪ A: Carcinoid tumor of the prostate. B: Carcinoid tumor of the prostate with cytoplasmic eosinophilic granules. |
Pathology
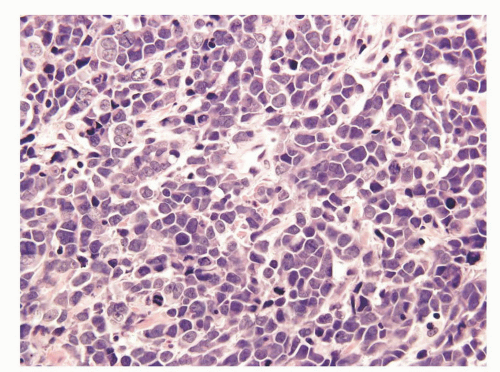
Prostatic and pulmonary small cell carcinomas share the same morphologic features.29 Classic “oat cell” morphology consists of tumor cells with scant cytoplasm and round, oval, or spindled nuclei usually smaller than three times the size of lymphocytes with fine granular chromatin, and absent or inconspicuous nucleoli, which is the histology seen in two-thirds of prostatic small cell carcinomas (Figs. 10-10 and 10-11). The remaining cases are of the “intermediate cell” variant with slightly more cytoplasm and more visible nucleoli (Fig. 10-12).30, 31, 32, 33 When diagnosing small cell carcinoma in the prostate, morphologic subtyping is not recommended and both subtypes are reported as “small cell carcinoma” as it has been demonstrated that in the lung these two types are clinically indistinguishable.34 Other features not typically associated with small cell carcinoma, including Indian file formation, giant cells with degenerative atypia, vacuolated cells, and desmoplasia, are seen in a minority of prostatic cases.28,35 Pure small cell carcinoma is seen in about one-half of cases with the remaining cases being admixed with prostate adenocarcinoma. In cases with adenocarcinoma, there is a sharp demarcation between small cell carcinoma and adenocarcinoma in about 20% of cases with the remaining cases showing a gradual merging of the two components (Fig. 10-13).28 Typically the associated acinar adenocarcinoma component is high-grade (Gleason score 8 to 10).
The vast majority (88%) of small cell carcinomas are positive for at least one neuroendocrine marker. In the small cell carcinoma component, 19% are positive for PSA, 28% for prostein (P501s), and 25% for PSMA, although often very focally. Although P501s and PSMA are better than PSA in identifying the prostatic origin of small cell carcinoma, the majority (60%) of prostatic small cell carcinomas are negative for all three markers. Stains for TTF are positive in about one-half of cases. Basal cell (p63, HMWK) and androgen
receptor markers are typically negative. About 50% of cases are AMACR positive. Small cell carcinoma is typically negative for both CK7 and CK20. Immunoreactivity with PAN-CK shows typical paranuclear dot-like pattern. Ki-67 staining shows >70% of positive cells. CD44 is reported to be positive in small cell prostatic carcinoma, but negative in small cell carcinoma of nonprostatic origin, and is expressed in only rare scattered cells in conventional adenocarcinoma.
receptor markers are typically negative. About 50% of cases are AMACR positive. Small cell carcinoma is typically negative for both CK7 and CK20. Immunoreactivity with PAN-CK shows typical paranuclear dot-like pattern. Ki-67 staining shows >70% of positive cells. CD44 is reported to be positive in small cell prostatic carcinoma, but negative in small cell carcinoma of nonprostatic origin, and is expressed in only rare scattered cells in conventional adenocarcinoma.
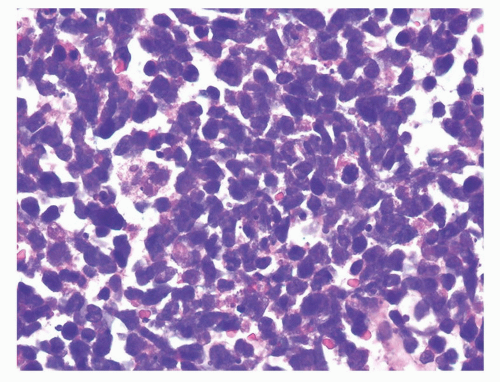 FIGURE 10-11 ▪ High magnification of small cell carcinoma of the prostate indistinguishable from small cell carcinomas in other sites. |
Differential Diagnosis
Small cell carcinoma must be differentiated from Gleason score 5 + 5 = 10 usual adenocarcinoma of the prostate. Cases where the small cell carcinoma component merges with non-small cell areas may be more likely to be underdiagnosed as opposed to cases whether there is a sharp demarcation between the two patterns.36 Also, the intermediate cell variant with more visible cytoplasm and nucleoli is at increased risk of a misdiagnosis of poorly differentiated usual prostate cancer.28 In part, the likelihood of underdiagnosing small cell carcinoma is due to the rarity of the diagnosis of small cell carcinoma relative to the diagnosis of usual prostate adenocarcinoma. Documentation of neuroendocrine differentiation using immunohistochemistry can help in cases that are ambiguous based on routine hematoxylin and eosin (H&E)-stained sections. It should be noted, however, that synaptophysin can show focal staining in high-grade acinar adenocarcinoma, and therefore the diagnosis should not be entirely based on the expression of neuroendocrine markers. Features that favor high-grade adenocarcinoma over small cell carcinoma are single cell pattern of growth, abundant cytoplasm, prominent nucleoli, absence of nuclear spindling and molding, strong positivity for prostatic markers, negative or focal staining for CD44, <50% of positive cells with Ki-67, and diffuse positivity for PAN-CK markers in contrast to small cell carcinoma in which the staining is dot-like paranuclear.
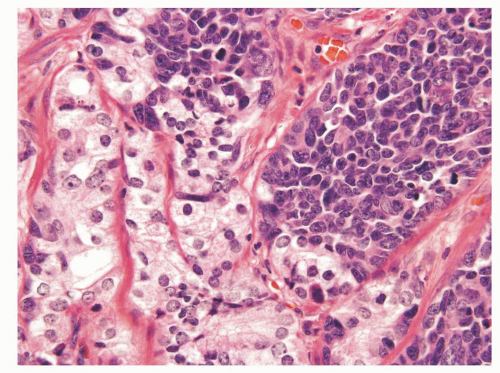 FIGURE 10-13 ▪ Small cell carcinoma with sharp demarcation between it and acinar adenocarcinoma of the prostate. |
In general, most cases of small cell carcinoma can be diagnosed on morphologic grounds alone, and immunohistochemistry is reserved for equivocal cases.
Prognosis and Treatment
Over 90% of small cell carcinomas are of advanced stage at time of diagnosis. In most cases with mixed usual adenocarcinoma of the prostate and small cell carcinoma, the metastases are of the small cell component. Treatment consists of combination chemotherapy of the type used in pulmonary small cell carcinoma. The prognosis is dismal with most surviving <2 years.37 There is no difference in prognosis whether tumors are mixed small cell and usual adenocarcinoma or pure small cell carcinoma. The proportion of small cell carcinoma and whether there is a history of prior usual adenocarcinoma of the prostate also does not affect prognosis. A review of the literature by Mackey et al.38 of genitourinary small cell carcinoma found cisplatin chemotherapy to be beneficial for bladder tumors but only surgery was prognostic for prostate small cell carcinomas. While this study concluded that hormonal manipulation and systemic chemotherapy had little effect on the natural history of disease in the prostate, the number of patients was small and some suggest that small cell carcinoma of the prostate should be treated with the same combination chemotherapy used to treat small cell carcinomas in other sites.39, 40, 41
Large Cell Neuroendocrine Carcinoma
Large cell neuroendocrine carcinoma (LCNEC) of prostate is an extremely rare occurrence.42 Most cases represent progression from prior typical prostate adenocarcinoma following long-standing hormonal therapy, with a median time interval of 4.7 years (range from 2 to 12 years) between the two. The tumors are composed of sheets and ribbons of amphophilic cells with large nuclei, coarse chromatin, and prominent nucleoli with a high mitotic activity and foci of necrosis (Fig. 10-14). The LCNEC component is strongly
positive various neuroendocrine markers with focal or absent PSA and PSAP immunoreactivity. AMACR may be positive. LCNEC are aggressive tumors that usually present at advanced stage and metastatic disease with patients dying within a year of diagnosis. The importance of distinguishing LCNEC from small cell carcinoma at the therapeutic and prognostic levels is not established. The diagnosis of LCNEC is problematic as usual prostate adenocarcinoma can express neuroendocrine markers. Only when a poorly differentiated carcinoma has a nested or ribbon-like appearance without glands, where the tumor is strongly positive for neuroendocrine markers and negative for prostate markers, can the diagnosis be established.
positive various neuroendocrine markers with focal or absent PSA and PSAP immunoreactivity. AMACR may be positive. LCNEC are aggressive tumors that usually present at advanced stage and metastatic disease with patients dying within a year of diagnosis. The importance of distinguishing LCNEC from small cell carcinoma at the therapeutic and prognostic levels is not established. The diagnosis of LCNEC is problematic as usual prostate adenocarcinoma can express neuroendocrine markers. Only when a poorly differentiated carcinoma has a nested or ribbon-like appearance without glands, where the tumor is strongly positive for neuroendocrine markers and negative for prostate markers, can the diagnosis be established.
Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinomas are very rare tumors with <10 cases reported to date. The mean age at presentation is 76 and patients usually present with urinary obstructive symptoms and elevated serum PSA levels.43 Histologically the lymphoepithelioma-like component is composed of sheets of undifferentiated cells with indistinct cytoplasmic borders and a syncytial pattern of growth. The nuclei are high-grade with vesicular chromatin, prominent nucleoli, and numerous mitoses. Typically there is an admixed heavy lymphocytic infiltrate with occasional plasma cells, neutrophils, and eosinophils. The lymphoepithelioma-like component ranges from 10% to 100% of the entire tumor and most cases have an associated acinar adenocarcinoma (most reported with Gleason score 4 + 3) and sometimes ductal adenocarcinoma components. Cells are positive for prostatic markers and AMACR and, when tested, in situ hybridization showed absence of Epstein-Barr virus genome confirming that this is a simply a rare morphologic variant of prostatic carcinoma. All reported cases were associated with an adverse clinical course with death from the disease occurring at a mean follow-up time of 20 months in the largest reported series.43
Paraganglioma
A few case reports of paragangliomas originating in the prostate have also been reported, including one in a child (Fig. 10-15).44, 45, 46
Urothelial Carcinoma
Introduction
Urothelial carcinoma can involve the prostate in the following different manners:
Urothelial neoplasia involving urethra, which may be seen in transurethral resection of the prostate (TURP) specimens. Those can be divided into
Urethral carcinoma in situ (CIS) in the absence of prostatic duct or stromal involvement.
Urothelial papillary neoplasms of the prostatic urethra: those lesions range from papilloma to high-grade noninvasive urothelial carcinoma to invasive papillary urothelial carcinoma involving the prostate.
Urothelial neoplasia involving prostatic ducts, acini, or stroma, which may be seen in TURP or needle biopsy specimens. Those can be divided into
CIS involving ducts and acini without stromal invasion (intraductal urothelial carcinoma).
CIS involving ducts and acini with stromal invasion.
Invasive urothelial carcinoma of the bladder with direct invasion of the prostatic stroma.
Clinical Features
The most common scenario for the detection of urothelial carcinoma of the prostate is prostatic involvement in cystoprostatectomies performed for bladder urothelial carcinomas. In that context the most common pattern of involvement is by extension of CIS to the prostatic urethra with secondary prostatic ducts/acinar involvement, which can progress to stromal invasion. A less common pattern of involvement is in advanced invasive bladder urothelial carcinomas that extend directly into the prostate. This latter scenario represents the pathologic stage T4 of the latest bladder TNM staging system. Random sections of the prostate at the time of cystectomy for urothelial carcinoma show prostatic involvement by urothelial carcinoma in between 12% to 20% of cases. If serial sections of the prostate in cystoprostatectomy specimens with bladder urothelial carcinoma are performed, involvement of the prostate by urothelial carcinoma may be found in 37% to 45% of the cases.47, 48, 49
Primary prostatic urothelial carcinoma is uncommon and comprises about 2% of prostate cancers in adults. The vast majority of those patients have concomitant bladder urothelial CIS, and prostatic involvement appears to be by direct extension from the overlying urethra, since in the majority of cases the more centrally located prostatic ducts are involved by urothelial neoplasia to a greater extent than the peripheral ducts and acini. However, rarely, primary urothelial carcinomas arise in the prostate without bladder involvement.47
Since topical chemotherapy and immunotherapy for superficial bladder carcinomas appear to act by direct contact with neoplastic epithelium, it has become critical to identify those cases of bladder urothelial carcinomas with prostatic involvement, since conservative management will not treat these cases effectively. Currently, biopsies of the prostatic urethra and suburethral prostate tissue may be performed as a staging procedure or in the setting of positive cytology in patients undergoing intravesical treatment for superficial bladder tumors. It is also important to evaluate the urothelium in routine TURP specimens done for benign prostatic hyperplasia (BPH) symptoms, as we have seen several cases of CIS where no history of bladder cancer was present.
Pathology
Urethral CIS in the absence of prostatic duct or stromal involvement: In those lesions, prostatic urethra is replaced by CIS cells. CIS can exhibit different patterns including large cell pleomorphic, large cell nonpleomorphic, small cell, clinging, and pagetoid. Criteria to
distinguish CIS from reactive urothelium are the same as those applied in the bladder.
Urothelial papillary carcinoma of the prostatic urethra: with low- or high-grade histology. Those lesions are usually noninvasive but can show submucosal invasion and even prostatic stromal invasion. For those invasive tumors, the TNM staging system of the urethra rather than the bladder applies and the pathologic stage is T1 for submucosal invasion and T2 for prostatic stromal invasion. T2 tumors are easily diagnosed when invasive tumor is seen infiltrating between prostatic glands; however, the distinction between T1 and T2 lesions when the involved tissue does not contain prostatic glands is not always straightforward due to the lack of clear-cut criteria to distinguish between the two different stages.
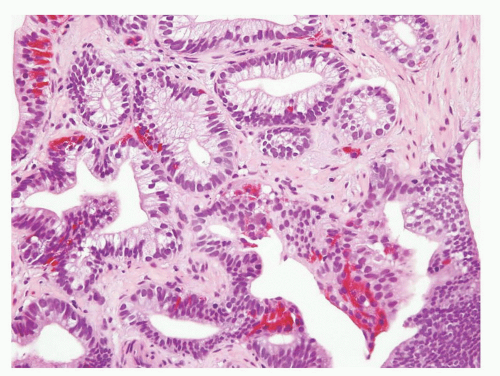
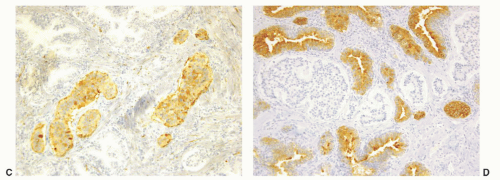
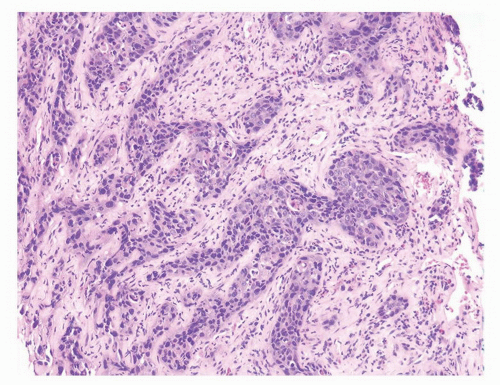
CIS involving ducts and acini without stromal invasion (intraductal urothelial carcinoma): Intraductal urothelial carcinoma of the prostatic ducts initially consists of malignant urothelial cells insinuating themselves between the basal cell layer and the columnar to cuboidal luminal epithelium of the prostatic ducts. More peripherally, urothelial carcinoma spreads in a pagetoid fashion within the ducts. Similar to that seen in the breast, large tumor cells with clear cytoplasm are seen in the midst of otherwise normal urothelium. With more extensive involvement, urothelial carcinoma fills and expands ducts and often develops central comedonecrosis (Fig. 10-16). Immunohistochemical stains for basal cells (HMWK, p63) may in some cases outline only the prostatic basal cells and in other cases also label the intraductal urothelial carcinoma. Sometimes the surrounding basal cells may show more intense staining for HMWK than the intraductal carcinoma component (Figs. 10-17 and 10-18).
CIS involving ducts and acini with stromal invasion: Stromal invasion is characterized by the presence of infiltrative small nests and single cells that usually elicit stromal inflammation and desmoplasia and may be accompanied by stromal retraction artifact. Those features along with
the irregular nests’ borders are helpful in the distinction between the invasive and the intraductal components. When urothelial carcinoma invades the prostatic stroma through this route, the urethral tumors staging system applies and the pathologic stage is considered to be T2.
Invasive urothelial carcinoma of the bladder with direct invasion of the prostatic stroma: These cases usually present with invasive urothelial carcinoma invading the prostatic stroma in the absence of an intraductal component or urethral involvement. Infiltrating urothelial carcinoma typically has a different morphology than poorly differentiated adenocarcinoma of the prostate, although there are some cases that show overlap and are impossible to distinguish without ancillary studies. Urothelial cancer tends to grow in nests, even when poorly differentiated (Fig. 10-19). High-grade urothelial carcinomas often reveal marked pleomorphism with tumor giant cells (Fig. 10-20). Urothelial carcinomas may demonstrate hard glassy eosinophilic cytoplasm or more prominent squamous differentiation. Urothelial carcinoma involving the prostate may contain areas of necrosis or stromal inflammation, which are unusual findings in even high-grade adenocarcinoma of the prostate. The pathologic stage of those tumors is T4 using the bladder TNM staging system.
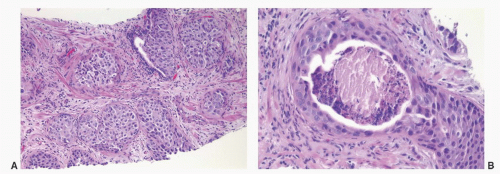 FIGURE 10-16 ▪ A: Intraductal spread of urothelial carcinoma. B: Urothelial carcinoma involving prostatic acini with central comedonecrosis. |
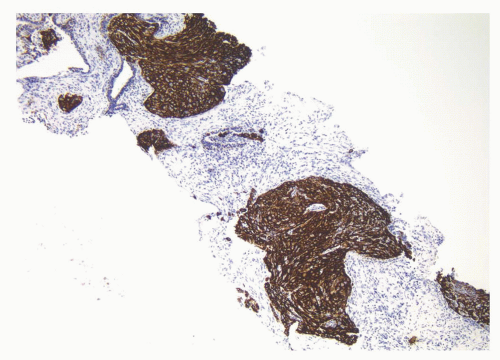 FIGURE 10-17 ▪ Intraductal urothelial carcinoma diffusely positive for high molecular weight cytokeratin. |
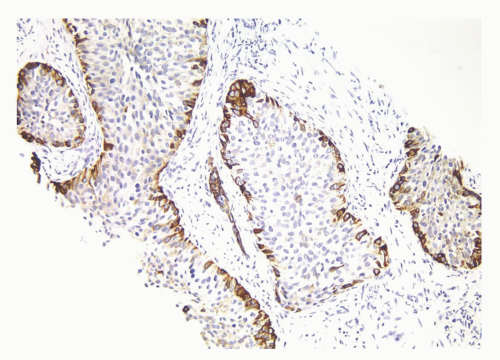 FIGURE 10-18 ▪ Intraductal urothelial carcinoma negative for high molecular weight cytokeratin with positivity restricted to residual benign prostatic basal cells. |
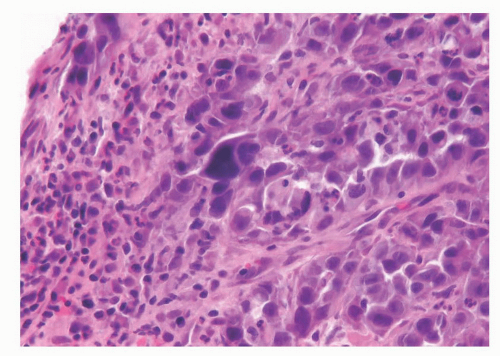 FIGURE 10-20 ▪ Urothelial carcinoma with marked pleomorphism and associated inflammation invading the prostate. |
Table 10-1 ▪ MORPHOLOGIC DISTINCTION OF POORLY DIFFERENTIATED PROSTATIC ADENOCARCINOMA FROM UROTHELIAL CARCINOMA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Differential Diagnosis
The major differential diagnosis for infiltrating urothelial carcinoma involving the prostate is poorly differentiated prostatic adenocarcinoma (Table 10-1). Poorly differentiated prostate cancers may have enlarged nuclei and prominent nucleoli, yet in contrast to urothelial carcinoma there is little variability in nuclear shape or size from one nucleus to another. A subtler finding is that the cytoplasm of prostatic adenocarcinoma is often very foamy and pale imparting a “soft” appearance. Infiltrating cords of cells or focal cribriform or pseudorosette glandular differentiation are other features more typical of prostatic adenocarcinoma than urothelial carcinoma (Fig. 10-21).
Although the above distinction between urothelial carcinoma and prostatic adenocarcinoma on H&E-stained sections is valid for almost all cases, we have seen rare cases where prostate adenocarcinoma has marked pleomorphism identical to urothelial carcinoma. Consequently, in a poorly differentiated tumor involving the bladder and prostate without any glandular differentiation typical of prostate adenocarcinoma, the case should be worked up immunohistochemically. Approximately 95% of poorly differentiated prostatic adenocarcinomas show PSA and PSAP staining although it may be focal.50, 51, 52 While some studies claim superiority of PSA over PSAP in staining prostatic carcinoma, other articles have demonstrated poorly differentiated prostatic carcinomas that lacked PSA staining but still maintained their immunoreactivity with antibodies to PSAP.51, 52, 53, 54 In our own hands, PSA has in general been more sensitive. Monoclonal antibodies to PSAP have lower sensitivities than their polyclonal counterparts.55 In some cases of poorly differentiated prostatic adenocarcinomas, there may be weak or negative PSA staining where the tumor reacts to a greater degree with newer prostate-specific markers including PSMA, p501S (prostein), and NKX 3.156 (Table 10-2). However, the lack of immunoreactivity to prostate-specific markers in a poorly differentiated tumor within the prostate, especially if present in limited amount, does not exclude the diagnosis of a poorly differentiated prostatic adenocarcinoma. In a poorly differentiated tumor occurring in the bladder and the prostate where the differential diagnosis is between high-grade prostatic adenocarcinoma and urothelial carcinoma, focal strong staining for prostatic markers can be used reliably to make the diagnosis of prostatic adenocarcinoma, as they have not been convincingly described in urothelial carcinomas.55,57,58 Almost 50% of cystoprostatectomy specimens performed for urothelial carcinoma also contain adenocarcinoma of the prostate.47,48,59 Therefore, the finding of a small focus of well-differentiated adenocarcinoma of the prostate in a TURP should not necessarily influence whether a separate focus of poorly differentiated tumor is urothelial carcinoma or adenocarcinoma of the prostate. In general, various cytokeratins (CK7, CK20, high molecular weight cytokeratin) show strong positivity in cases of urothelial carcinoma involving the prostate. Although CK7 and CK20 are more frequently seen in urothelial carcinoma as compared to adenocarcinoma of the prostate, they may also be positive in adenocarcinoma of the prostate.60,61 The only helpful finding is negative CK7 immunoreactivity, which favors prostatic adenocarcinoma, as it would be unusual for urothelial carcinoma. High molecular weight cytokeratin is positive in more than 90% of urothelial carcinomas.56,62 In contrast, high molecular weight cytokeratin is only rarely (8%) expressed, and usually in a very small percentage of cells, in adenocarcinoma of the prostate.56,63 p63 has a greater specificity albeit lower sensitivity for urothelial carcinoma compared to high molecular weight cytokeratin (100% specificity and 83% sensitivity).56 Other markers that also appear highly specific but only of modest sensitivity for urothelial carcinoma include uroplakin and thrombomodulin (49% to 69% sensitivity).
Although the above distinction between urothelial carcinoma and prostatic adenocarcinoma on H&E-stained sections is valid for almost all cases, we have seen rare cases where prostate adenocarcinoma has marked pleomorphism identical to urothelial carcinoma. Consequently, in a poorly differentiated tumor involving the bladder and prostate without any glandular differentiation typical of prostate adenocarcinoma, the case should be worked up immunohistochemically. Approximately 95% of poorly differentiated prostatic adenocarcinomas show PSA and PSAP staining although it may be focal.50, 51, 52 While some studies claim superiority of PSA over PSAP in staining prostatic carcinoma, other articles have demonstrated poorly differentiated prostatic carcinomas that lacked PSA staining but still maintained their immunoreactivity with antibodies to PSAP.51, 52, 53, 54 In our own hands, PSA has in general been more sensitive. Monoclonal antibodies to PSAP have lower sensitivities than their polyclonal counterparts.55 In some cases of poorly differentiated prostatic adenocarcinomas, there may be weak or negative PSA staining where the tumor reacts to a greater degree with newer prostate-specific markers including PSMA, p501S (prostein), and NKX 3.156 (Table 10-2). However, the lack of immunoreactivity to prostate-specific markers in a poorly differentiated tumor within the prostate, especially if present in limited amount, does not exclude the diagnosis of a poorly differentiated prostatic adenocarcinoma. In a poorly differentiated tumor occurring in the bladder and the prostate where the differential diagnosis is between high-grade prostatic adenocarcinoma and urothelial carcinoma, focal strong staining for prostatic markers can be used reliably to make the diagnosis of prostatic adenocarcinoma, as they have not been convincingly described in urothelial carcinomas.55,57,58 Almost 50% of cystoprostatectomy specimens performed for urothelial carcinoma also contain adenocarcinoma of the prostate.47,48,59 Therefore, the finding of a small focus of well-differentiated adenocarcinoma of the prostate in a TURP should not necessarily influence whether a separate focus of poorly differentiated tumor is urothelial carcinoma or adenocarcinoma of the prostate. In general, various cytokeratins (CK7, CK20, high molecular weight cytokeratin) show strong positivity in cases of urothelial carcinoma involving the prostate. Although CK7 and CK20 are more frequently seen in urothelial carcinoma as compared to adenocarcinoma of the prostate, they may also be positive in adenocarcinoma of the prostate.60,61 The only helpful finding is negative CK7 immunoreactivity, which favors prostatic adenocarcinoma, as it would be unusual for urothelial carcinoma. High molecular weight cytokeratin is positive in more than 90% of urothelial carcinomas.56,62 In contrast, high molecular weight cytokeratin is only rarely (8%) expressed, and usually in a very small percentage of cells, in adenocarcinoma of the prostate.56,63 p63 has a greater specificity albeit lower sensitivity for urothelial carcinoma compared to high molecular weight cytokeratin (100% specificity and 83% sensitivity).56 Other markers that also appear highly specific but only of modest sensitivity for urothelial carcinoma include uroplakin and thrombomodulin (49% to 69% sensitivity).
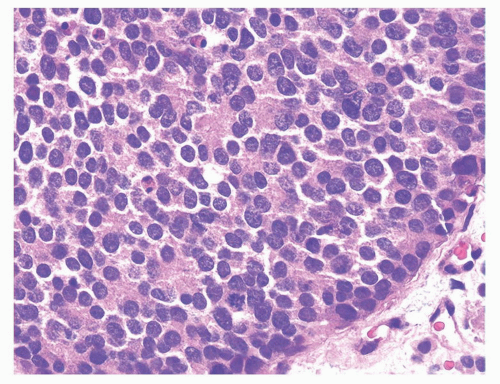 FIGURE 10-21 ▪ High-grade acinar adenocarcinoma of the prostate with uniform cytology and subtle microacinar formation. |
Table 10-2 ▪ IMMUNOHISTOCHEMICAL DISTINCTION OF PROSTATE ADENOCARCINOMA AND UROTHELIAL CARCINOMA | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Extensive intraductal urothelial carcinoma must be differentiated from invasive urothelial carcinoma. With intraductal urothelial carcinoma of the prostate, nests of urothelial carcinoma have the contours and distribution of prostatic ducts and acini. The nests are circumscribed with a smooth discrete edge between the epithelium and the adjacent stroma, and the stroma lacks a desmoplastic response. Infiltrating urothelial carcinoma is characterized by small cords, nests, or individual cells often with retraction artifact, eliciting a desmoplastic stromal response.
Intraductal urothelial carcinoma must also be differentiated from high-grade prostatic intraepithelial neoplasia. The presence of an intraductal growth where preexisting benign prostate glands are filled with solid nests of tumor differs from HGPIN, which is composed of flat, tufting, papillary, or cribriform patterns. Although the cells of HGPIN are atypical, the presence of pleomorphism and mitoses are uncommon.
Prognosis and Treatment
Patients with prostatic stromal involvement by direct invasion of the bladder urothelial carcinoma (T4) have a worse prognosis than those in whom the invasive prostatic urothelial carcinoma originates from an intraductal component.64 In the latter scenario, there are typically associated bladder tumors, which may determine the prognosis. Intraductal prostatic urothelial carcinoma with prostatic stromal invasion may also be associated with low-stage (T1, T2) bladder tumors. Prostatic stromal invasion in this setting has a negative effect on prognosis, although the prognosis is less ominous than direct invasion into the prostate from an infiltrating bladder urothelial carcinoma.64, 65, 66
Most but not all studies have demonstrated that intraductal spread of the prostate by urothelial carcinoma does not adversely affect survival following cystoprostatectomy, which is rather determined by the stage of the bladder tumor.49,64, 65, 66, 67 If intraductal urothelial carcinoma is identified on TURP or transurethral biopsy, patients usually will be recommended for radical cystoprostatectomy. The finding of intraductal urothelial carcinoma has also been demonstrated to increase the risk of urethral recurrence following cystoprostatectomy, such that its identification may also result in prophylactic total urethrectomy.
The overall prognosis of urothelial cell carcinoma diagnosed on prostatic needle biopsy is poor, even in cases without histologic evidence of stromal invasion on biopsy.63 In these cases with intraductal cancer on biopsy, most likely invasive cancer is present elsewhere in the prostate that was not sampled. Although the prognosis is poor, even with only apparent intraductal involvement, histologic recognition is essential, as the only opportunity for improved outcome is early and aggressive therapy.
Primary urothelial carcinoma of the prostate without bladder involvement is rare.68, 69, 70




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree