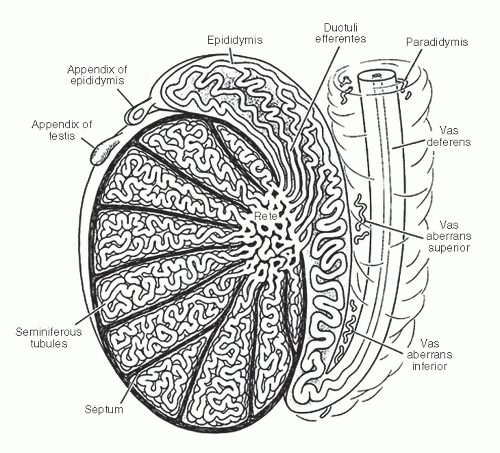
and basal cells, the former containing long stereocilia. The luminal contour of the vas is variably folded, and the epithelium is surrounded by loose connective tissue and a very thick smooth muscle layer.
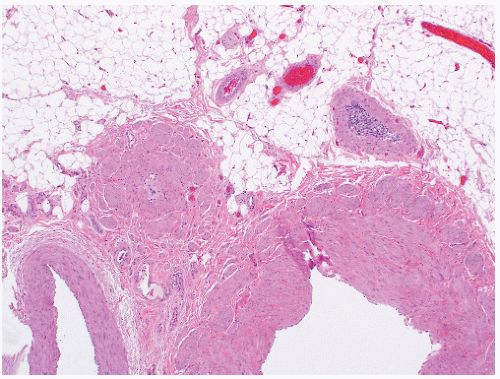
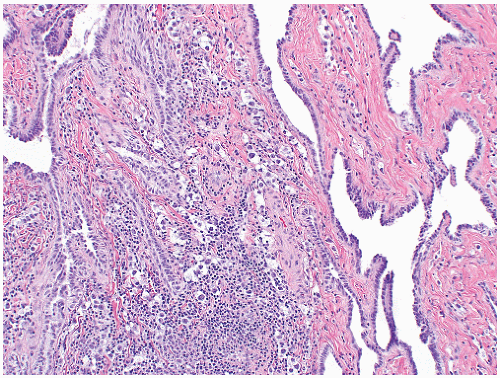
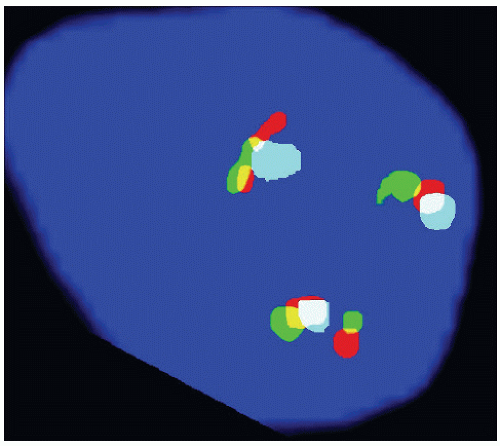
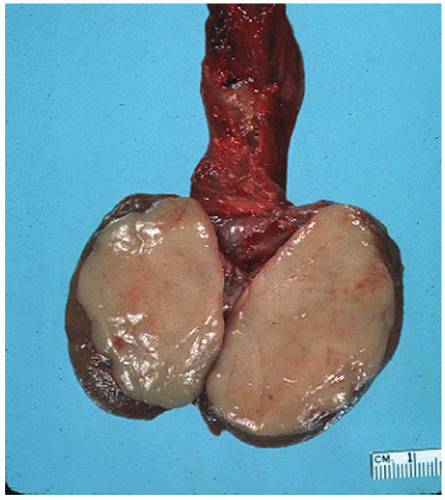
of the gonad flows toward the rete testis/mediastinum testis as well as toward the tunica albuginea (Figs. 12-3A and B). For this reason, sections that include tumor and overlying tunica as well as tumor with adjacent rete testis/mediastinum testis are particularly informative. Sections of the testicular adnexa (efferent tubules and epididymis) should be taken although involvement of these structures by tumor does not affect pathologic stage and does not seem to affect prognosis. This also applies to tumor involvement of soft tissue in the mediastinum, in the absence of lymphovascular invasion. A recent publication suggests that both rete testis involvement and hilar fat involvement are independently associated with advanced disease at presentation, but the study does not address the issue of association with relapse in clinical stage 1 disease17 (Fig. 12-4). We suggest that at
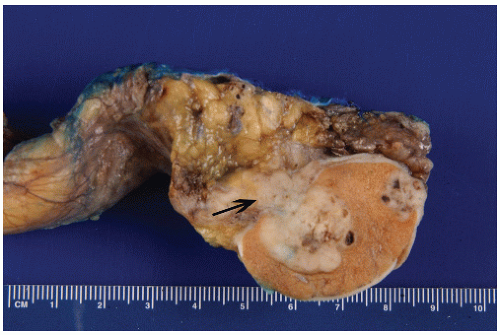
least three sections of the spermatic cord be taken: proximal (margin of resection), mid, and distal (near the adnexa). Involvement of the soft tissue of the spermatic cord is considered pathologic stage 3 and clinical stage 2 disease and is commonly associated with the presence of metastatic disease at the time of orchiectomy. Of note, intravascular or intralymphatic involvement within any part of the spermatic cord is still considered pT2 (Fig. 12-5).
Table 12-1A ▪ TNM CLASSIFICATION OF GCTs OF THE TESTIS | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Table 12-1B ▪ CLINICAL STAGING OF GCTs OF THE TESTIS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 12-1C ▪ SERUM TUMOR MARKERS IN TESTICULAR GCTs* | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
on multivariate analysis, at least one recent large study that included 687 stage 1 tumors puts this belief into question18 (Fig. 12-6).
been diagnosed in the United States in 2013 with only 370 dying of disease33 (Table 12-3). Because of their relative rarity, they present a diagnostic challenge to most practicing pathologist (Box 12-1).
Table 12-2 ▪ WHO HISTOLOGIC CLASSIFICATION OF TESTIS TUMORS* | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
There appears to be a geographic and racial predisposition to the development of testicular GCTs with a twofold increase in Scandinavian countries as compared to the United States and a lower incidence in other countries such as Africa and some Latin American countries such as Puerto Rico.39 Patients with prior testicular GCT are more likely to develop a contralateral tumor than does the general population.
Table 12-3 ▪ IMMUNOHISTOCHEMICAL PROFILE OF TESTICULAR TUMORS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
in these high-risk patients to detect incipient neoplasia. In a large series, only one of more than 1,500 cryptorchid patients with testicular biopsy specimens negative for IGCNU developed testicular cancer over a follow-up period of 8 years,48 in contrast to 50% of patients with IGCNU who developed invasive tumors over a 5-year period.49
Table 12-4 ▪ TESTICULAR GERM CELL TUMORS | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
developed invasive GCT at this site within the follow-up period. Mathematical modeling suggested that 50% of biopsy-positive cases would develop disease within 5 years. Remarkably, not a single case of contralateral GCT developed in the remaining 463 biopsy-negative patients during the same follow-up period. In a subsequent report the authors revealed that at least two of the biopsy-positive cases that received systemic therapy subsequently developed contralateral tumors, suggesting that systemic therapy is not always effective against preinvasive disease.65
|
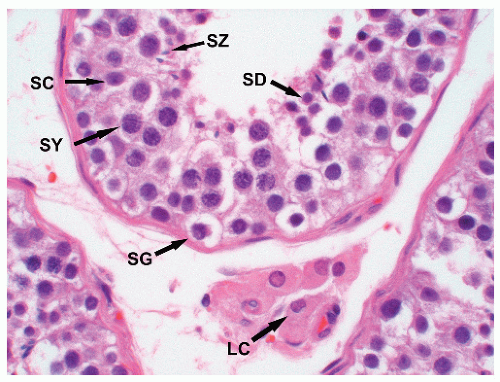
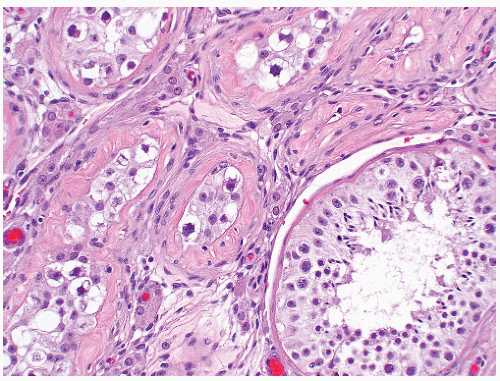
chromatin, and absent nucleoli. In most cases, tumor-bearing tubules do not have active spermatogenesis and contain mostly Sertoli cells. Sertoli cells may be displaced toward the tubular lumen. Characteristically, they contain a single nucleolus that is small and regular. The nuclei are oval or round with regular borders and the chromatin is fine. The cytoplasm is amphophilic/eosinophilic and not vacuolated.
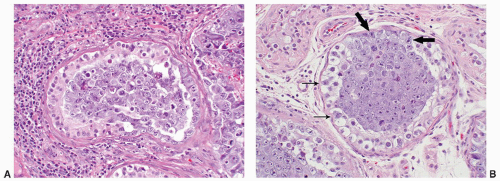
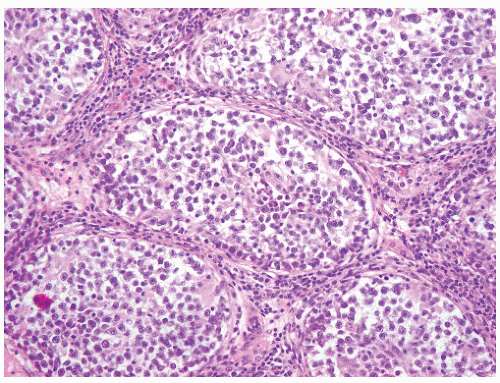 FIGURE 12-10 ▪ Seminoma cells fill the lumen of a seminiferous tubule. This should not be called intratubular germ cell neoplasia. |
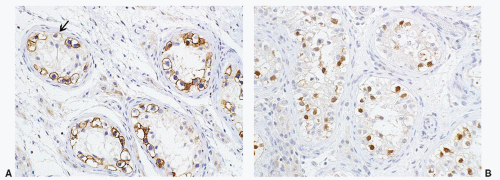
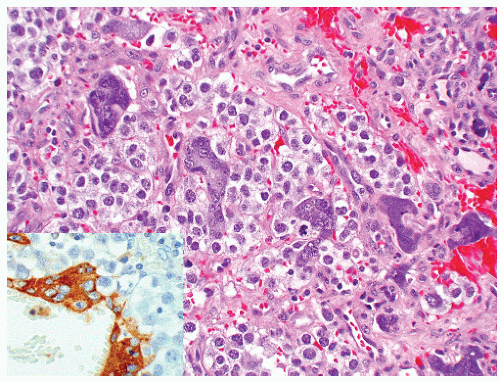
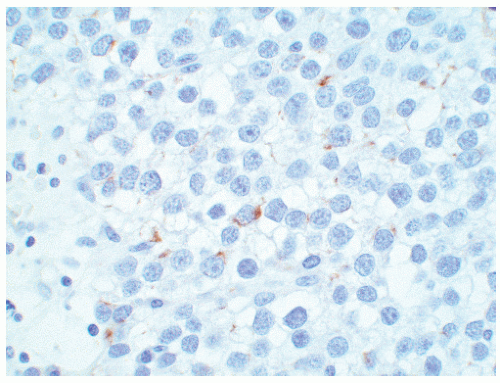
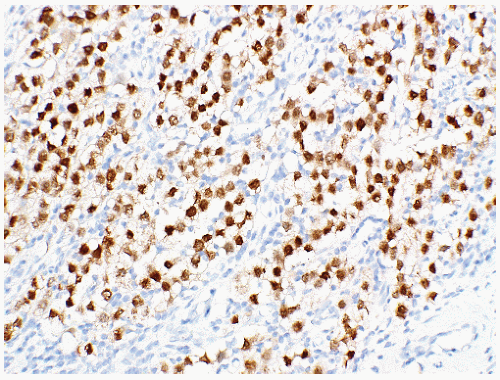
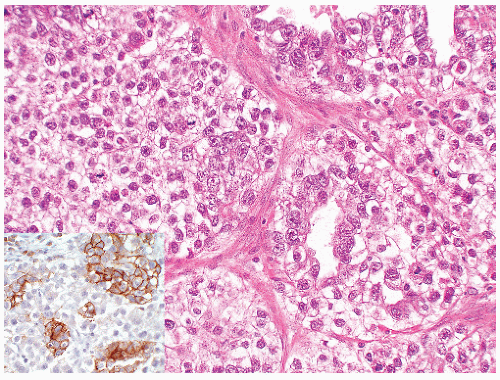
seen in other types of non-germ cell malignancies.70, 71, 72, 73 C-kit (CD 117) is expressed in a large percentage of IGCNU as well as seminomas, but not in other GCTs.74 Once again, the staining pattern is cytoplasmic/membranous (Fig. 12-13A). Despite the overexpression of this antigen, C-kit is rarely mutated in these tumors. Other antibodies that immunoreact with IGCNU but are rarely used in clinical practice include M2A and 43-F.73,75,76 POU5F1 (Oct3/4) is a very interesting marker with great clinical utility.77 The gene serves as a transcription factor and its product is expressed in pluripotent mouse and human embryonic stem cells and is downregulated during differentiation. Since the gene is also required for self-renewal of embryonic stem cells, knocking out the gene is lethal. This antigen is expressed solely in IGCNU, seminoma, and EC, suggesting that these are the types of GCT cells with pluripotency, that is, with the capacity to differentiate. As a transcription factor, staining is localized to the nucleus (Fig. 12-13B).
Table 12-5 ▪ GCTs RISK CLASSIFICATION: INTERNATIONAL CONSENSUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
must adhere strictly to the established diagnostic criteria. On occasion spermatogonia may be enlarged and notably hyperchromatic, superficially mimicking ICGNU at low magnification. These changes are thought to be degenerative and are not associated with the development of tumor. These cells can be easily seen at intermediate to high magnification since they lack the characteristic coarse chromatin, nuclear contour irregularities, and prominent nucleoli seen in ICGNU. As you might expect, the immunoprofile of these cells is quite different, lacking expression of PLAP and OCT4. At times, spermatogonia can express C-kit and SALL4. Proper care must be taken to examine closely the cytologic features of the immunoreactive cells. While some investigators have reported PLAP immunoreactivity in rare spermatogonis, we have not encountered this problem. Besides intratubular seminoma, one can encounter intratubular EC (Fig. 12-14A and B), intratubular SS, and even metastatic disease such as melanoma and prostatic carcinoma. Intratubular lymphoma and even mesothelioma may also be confused with IGCNU.
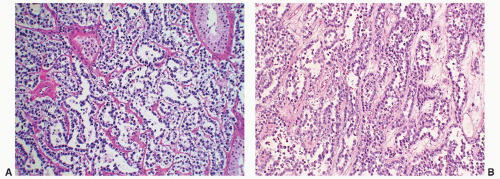
serum levels of β-HCG. Patients with metastases are more likely to have elevated levels of β-HCG; however, elevation of this marker does not appear to be an adverse prognostic marker but rather a marker of bulkier disease or advanced stage.89 AFP levels generally are not elevated in pure seminoma, and any elevations require more thorough sampling of the tumor for a nonseminomatous component. LDH can be elevated in seminoma but this elevation is in no way specific to this disease but generally is a marker of the extent of disease; the larger the tumor burden, the high the serum levels.
|
Seminomas seen in association with a parenchymal scar should bring to mind the possibility of partial regression of the tumor, which may have been seminoma or possibly another GCT component other than teratoma. This phenomenon may be the explanation in some cases for nonseminomatous metastases in otherwise pure testicular seminomas. Whenever a parenchymal scar suggestive of partial regression is encountered, it is important to include it in the pathology report as a comment since this finding may account for the discrepancy between the pathology of the viable tumor and serum tumor markers.
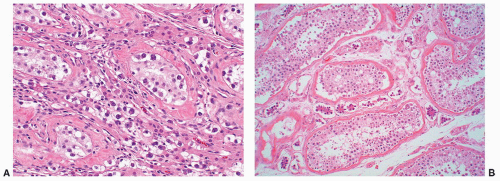
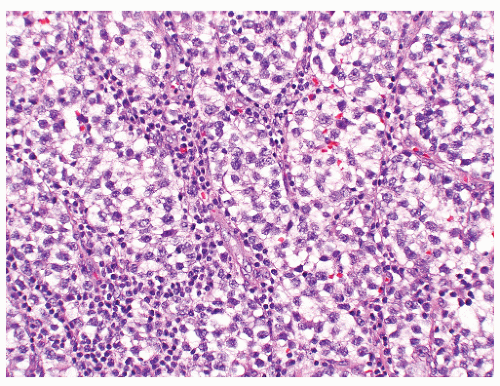 FIGURE 12-16 ▪ Seminoma, classic type. Cells have squaredoff nuclei, clear cytoplasm with little nuclear overlap. Mature lymphocytes are present in the septa. |
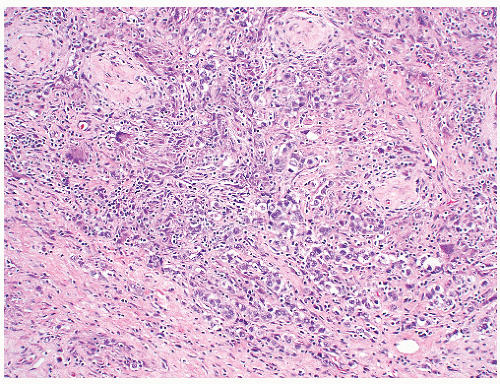
of “anaplastic seminoma” as a discreet entity with a worse prognosis.31,112 As described, this tumor was characterized by overall morphologic features of a seminoma but containing more pleomorphic cells with nonclear cytoplasm and abundant mitotic figures. Fibrovascular septa and lymphocytes were absent and focal necrosis was commonly seen. This concept did not withstand the test of time since many series later showed that stage for stage there was no difference in clinical outcome between classic and anaplastic seminomas.26,103,113 In addition, it has become quite evident that mitotic activity in seminomas is quite variable and, in fact, may be quite high even in classical cases.105 For the last decade or so, we have used the term “seminoma with atypia” not as a diagnostic entity but rather as a reminder to consider all possible explanations for the atypical morphologic findings (Fig. 12-23).
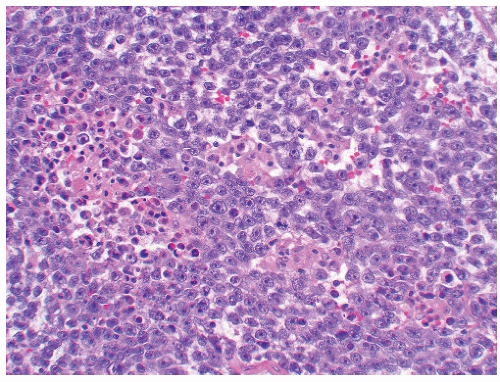 FIGURE 12-23 ▪ Seminoma with atypia. While some tumors may lack a lymphocytic infiltrate and exhibit atypical cytologic features, one must consider other possibilities in the differential diagnosis. |
phenotype. Others would not change management based on this finding alone since, in the absence of other adverse prognostic factors, the patient would likely be placed on surveillance either way. The fact of the matter is that decisions on how to treat these patients are at best empiric because of the rarity of the situation and the absence of clinical studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree