Chapter 25 Pancreatoscopy
Peroral pancreatoscopy, in which a small caliber fiberscope (“baby scope”) is inserted into the pancreatic duct from the papilla through the working channel of a duodenoscope (“mother scope”), was first described by the Japanese investigators Takagi and Takegoshi1 in 1974. Although the idea was very attractive and several investigators studied its feasibility, pancreatoscopy was not widely adopted because of low visibility and instrument fragility as well as the relatively large diameter of the instrument compared to the size of the papilla. The description of intraductal papillary mucinous neoplasm (IPMN) by another Japanese investigator, Ohashi et al.2, made an impact on pancreatoscopy in the early 1980s. Since then, pancreatoscopy has been reassessed as a useful modality for the diagnosis of IPMN showing characteristic endoscopic findings.
Video for this chapter can be found online at www.expertconsult.com.
Several recent developments have rekindled interest in pancreatoscopy. The first is the emergence of video (electronic) pancreatoscopes, which was first made by our group with the development of a miniature charge-coupled device (CCD) video chip in 1999.3 The second is the development of pancreatoscopy with adjunct imaging techniques such as narrow band imaging (NBI) and autofluorescence imaging (AFI). The third is the application of the SpyGlass Direct Visualization single-operator cholangiopancreatoscopy system in the pancreatic duct.
Description of Technique
Equipment
Pancreatoscope
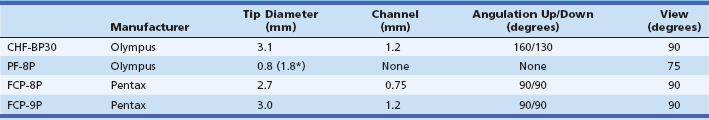
The most commonly used fiberoptic pancreatoscope has an outer diameter of more than 3 mm. It has both tip deflection and an accessory channel (more than 1.2 mm in diameter) that allows biopsy and lithotripsy under direct visualization.4,5 A second type of device, known as the ultrathin pancreatoscope, has an outer diameter of 0.75 or 0.8 mm and contains 3000 to 6000 image fibers.4,6 Since the ultrathin pancreatoscope can be inserted through a standard ERCP catheter, it can be applied easily at the time of ERCP. Although it has no tip deflection or accessory channel, cytology sampling as well as injection and aspiration of saline are available through the outer cannula. Specifications for representative fiberoptic pancreatoscopes are compared in Table 25.1.
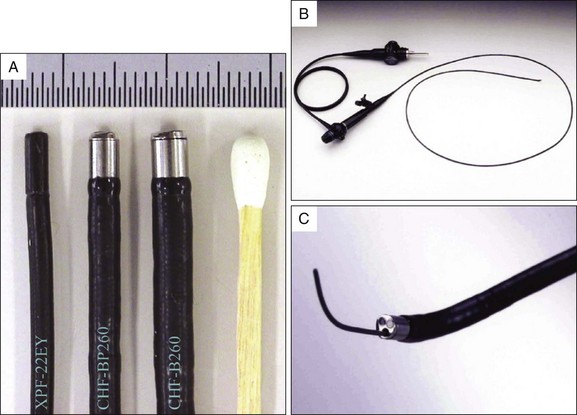
The video pancreatoscope was first described by Kodama et al.7 A first prototype was developed with a newly designed 50,000-pixel interline CCD (about 1 square mm in size) by Matsushita Electronics Corp. (Osaka, Japan) in cooperation with Olympus Optical Co. (Tokyo). The prototype (XPF-22EY) had an outer diameter of 2.1 mm and two-way tip deflection without an accessory channel. After the prototype’s success, Olympus developed an enhanced version that has an accessory channel (XCHF-BP240).8 At present Olympus offers two types of video pancreatoscopes in Japanese markets. The first type (CHF-BP260) is a commercial model of XCHF-BP240 with a 2.6-mm outer diameter and a 0.5-mm accessory channel. The second type (CHF-B260) is a larger scope with a 3.4-mm outer diameter and a 1.2-mm accessory channel that allows biopsy and lithotripsy under direct visualization (Fig. 25.1). These scopes use a field sequential imaging system. In the United States the larger video pancreatoscope (CHF-B160) using a simultaneous imaging system is available for special order. The specifications for the prototype and commercial video pancreatoscopes are compared in Table 25.2.
Table 25.2 Specifications of the Prototype and Commercially Available Video Pancreatoscopes (Olympus Medical Systems Co.)
These pancreatoscopes are inserted through the working channel of a duodenoscope (mother scope) as baby scopes. All baby scopes require a dedicated light source and image processor. The baby scope image is projected on a separate video monitor (Fig. 25.2).
Endoscopic Procedure
Using a two-operator method, one endoscopist operates the mother duodenoscope and the other operates the baby scope (Fig. 25.3). A duodenoscope is positioned at the ampulla and the baby scope is inserted through the working channel of the duodenoscope. A prior pancreatic sphincterotomy or sphincteroplasty is usually needed when inserting a relatively large pancreatoscope. Intubation with the baby scope is performed by the endoscopist who operates the duodenoscope. Because the baby scope is fragile at the bending part of its tip, care is taken to advance the baby scope with the elevator of the duodenoscope open. For baby scopes equipped with an accessory channel, inserting the baby scope over a guidewire reduces the need for elevator use and the risk of scope damage. The pancreatic duct is first cannulated with a guidewire, and the baby scope is then loaded over the guidewire. The baby scope should be advanced into the pancreatic duct with the aid of up angulation of the duodenoscope rather than the use of the elevator because of its fragility. Alternatively, for baby scopes that have no accessory channel, the scope must be inserted directly into the pancreatic duct.
Once in the pancreatic duct, the baby scope can be advanced deeply into the pancreatic duct under both direct endoscopic and fluoroscopic guidance. A torturous portion at the pancreas head is the most difficult part to pass. The baby scope is mainly steered by the endoscopist handling the duodenoscope, while repositioning the duodenoscope relative to the papilla. The endoscopist handling the baby scope can fine-tune the steering with tip deflection of the baby scope. Water irrigation is usually needed to optimize visualization. Protein plugs in the pancreatic duct may impair visualization and will need to be flushed out with sterile saline solution. For baby scopes that have no accessory channel, removal of the baby scope may be necessary to irrigate the duct with an ERCP cannula. Intravenous secretin (100 units) has been used to stimulate pancreatic juice flow in an effort to improve visualization of the pancreatic duct.7 To facilitate insertion of accessories, such as biopsy forceps or electrohydraulic lithotripsy (EHL) probe, the elevator of the duodenoscope needs to be relaxed and the angulation of both the duodenoscope and the baby scope need to be reduced.
Indications
Differentiation of Stenosis of the Main Pancreatic Duct (Benign or Malignant)
EUS has a high diagnostic yield in identifying pancreatic masses that may not be detected on noninvasive imaging. The addition of fine-needle aspiration (FNA) capability further enhances diagnostic accuracy for pancreatic cancer with a sensitivity ranging from 80% to 95%.9,10 The role of pancreatoscopy in cancer diagnosis has yet to be defined. However, pancreatoscopy offers the advantages of direct visualization and inspection of the ductal epithelium for subtle mucosal abnormalities or vascular patterns.
In a series of 52 patients (8 with pancreatic cancer, 19 with chronic pancreatitis, and 25 normal cases), 42 (81%) were observed successfully with the ultrathin pancreatoscope (0.8 mm).6 Pancreatoscopic findings in the seven malignant strictures were friability and erythema (100%), nodularity (71%), and erosive changes (57%). Strictures with scarred appearance were observed in 12 (80%) of 15 cases with chronic pancreatitis.
In another series of patients with 35 pancreatic cancers and 20 benign stenoses, 22 (63%) and 16 (80%) patients, respectively, were adequately seen with the ultrathin pancreatoscope.11 Findings specific to pancreatic cancer were friability (50%), protrusion (27%), tumor vessels (23%), and papillary tumor (14%). Stenosis without significant mucosal changes was observed in 62% of cases with benign stenosis. Coarse mucosa and friability were observed more frequently in association with pancreatic cancer than benign stenosis.
Carcinoma in situ of the pancreas can be diagnosed by pancreatoscopy. In a series of 11 patients with pancreatic carcinoma in situ, peroral pancreatoscopy with the ultrathin pancreatoscope and pancreatic juice sampling for cytology were performed preoperatively.12 Pancreatoscopy revealed the site of the lesion in 10 cases with carcinoma in situ in the main pancreatic duct. Findings of carcinoma in situ were papillary mucosa, irregular mucosa, or nodular mucosa. Using pancreatoscopic cytology, cancer cells were obtained from all lesions in the main pancreatic duct, while conventional pancreatic juice cytology was diagnostic in only 60% of the cases.
Intraductal Papillary Mucinous Neoplasm (IPMN)
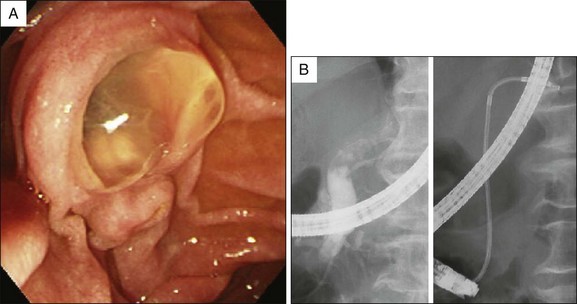
IPMN is the best indication for pancreatoscopy due to the patulous papilla and the dilated pancreatic duct (Fig. 25.4). The usefulness of peroral pancreatoscopy for IPMN has been underscored in several reports.13–20 In patients with IPMN and equivocal radiographic findings, pancreatoscopy may provide valuable information for the differential diagnosis of amorphous filling defects in the main pancreatic duct and may make possible a definite diagnosis based on the characteristic appearance of papillary tumors.21,22 A biopsy can be taken from abnormal-appearing mucosal lesions under direct vision when a pancreatoscope with an accessory channel is used. Pancreatoscopy may provide valuable information in assessing the extent of the lesion to select the best surgical procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree