Chapter 26 Cholangioscopy
Introduction
Advances in cholangiopancreatoscopy continue to broaden the interventionalist’s scope of effectiveness in the diagnosis and management of biliary and pancreatic disease. Continued design evolution has moved this technology from academic research centers to the hands of the practicing community. The nomenclature has likewise evolved through a spectrum of descriptive terms including cholangioscopy, choledochoscopy, duodenoscope-assisted cholangiopancreatoscopy, and peroral cholangioscopy, among others. The duct can be accessed through a variety of approaches: through the native papilla of Vater via a duodenoscope, percutaneously through a transhepatic route, or intraoperatively through a choledochotomy or cystic duct exploration. This chapter is divided into three parts. Part B focuses on the developing field of videocholangioscopy and the enhancements that can be expected as that technology becomes more available. Part C, on the Spyglass system, emphasizes the single-operator approach that has greatly simplified cholangioscopy, allowing for its expanded use.
Video for this chapter can be found online at www.expertconsult.com.
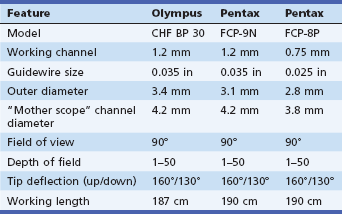
Cholangioscopes first appeared in research academic settings over 25 years ago and since then have slowly undergone a series of iterations. Currently, in the United States, there is a limited assortment of fiberoptic cholangioscopes available for purchase (Table 26.1). In general, the smaller the outer diameter of the cholangioscope, the greater its maneuverability within the bile duct. The trade-off, however, is that these smaller scopes may have a smaller working channel or fewer internal cables for tip deflection. The ultraslim scopes are of sufficiently small caliber that they can be introduced into either the biliary or the pancreatic ducts through standard catheters and deliver good visualization of small secondary ductal branches. Their lack of a working channel or flushing mechanism, however, is a disadvantage compared with the larger therapeutic cholangioscopes.
The optimal endoscopic retrograde cholangiopancreatography (ERCP) suite design places the cholangioscope, its power supply, and accessories in close proximity to the endoscopist. Because of the intimate spatial working relationships between the mother and baby scope, the cholangioscope components are set up on or adjacent to the ERCP processor cart. These components include the light generator and image processor as well as the flushing and suctioning equipment. Depending on the manufacturer and the model, the light source, image processing hardware, and air and fluid pump are available either as individual components or combined in a single unit. The Spyglass system, as discussed in Part C, combines all of these units on one cart.
To show how far we have come from the time when I first converted a two-person procedure to one that could be performed solo, refer to Fig. 26.1.
Videocholangioscopy
Videocholangioscopy Using the Mother-Baby System
A videocholangioscope can provide outstanding quality digital images compared to conventional fiberoptic cholangioscopy.1–9 Two videocholangioscopes are used in the mother-baby cholangioscopy system (Table 26.2). At the present time, however, their use is limited to a few countries.
Description of the Technique
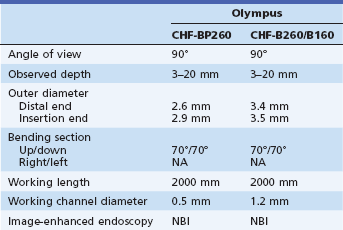
The therapeutic duodenoscope that is used as the mother scope has a 4.2-mm working channel, which helps prevent kinking of the baby videocholangioscope. Endoscopic sphincterotomy is needed to facilitate scope passage across the papilla. Two videocholangioscopes (CHF-B260/B160 and CHF-BP260, with outer diameters of 3.4 and 2.8 mm and working channel diameters of 1–2 and 0.8 mm, respectively; B260 and BP260, Olympus Medical Systems, Tokyo; B160, Olympus America Inc., Center Valley, Pa.) (Fig. 26.2) are advanced through the 4.2-mm working channel of the therapeutic duodenoscope into the bile duct with or without a 0.035-in guidewire. They have two-way tip angulation. The wire-guided insertion technique is used with the CHF-B260 but not with the CHF-BP260 because of the diameter of the working channel. Saline irrigation and carbon dioxide (CO2) insufflation are used for observation in the bile duct.7,8 Endoscopic observation is usually performed using white light imaging. Observation using narrow band imaging (NBI) is available with the NBI system (CV-260SL, CVL-260SL/CV-180, CLV-180, light source, Olympus).
Technique: Diagnostic and Therapeutic
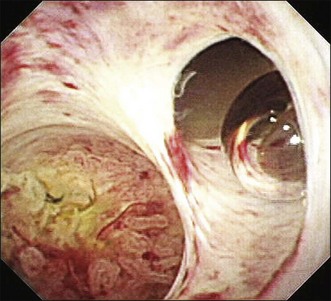
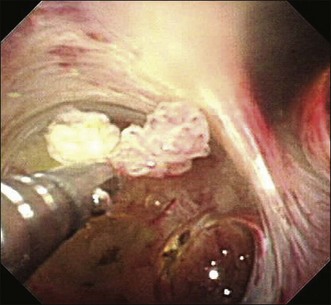
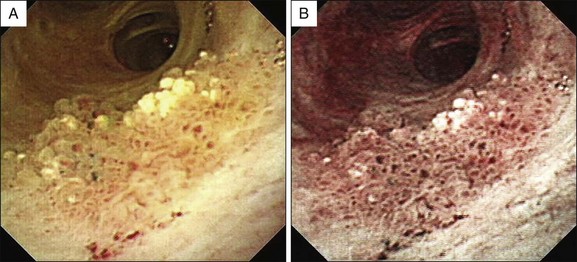
Videocholangioscopy provides better-quality digital images and offers enhanced mucosal detail compared with conventional fiberoptic cholangioscopes (Fig. 26.3; Video 26.1). Thus it can delineate fine mucosal structures like shallow pseudodiverticular, bumpy surface, papillary, or granular lesions and fine vessel patterns, leading to differentiation between benign and malignant lesions on the basis of the indeterminate filling defects and biliary strictures as described by cholangiography. A recent study of the ability of videocholangioscopy to differentiate between indeterminate filling defects and biliary strictures revealed that ERCP/tissue sampling and videocholangioscopy provide high diagnostic ability (accuracy 98.3%, sensitivity 99.0%, specificity 95.8%, positive predictive value 99.0%, negative predictive value 95.8%) compared with ERCP/tissue sampling alone (accuracy 85.0%, sensitivity 86.5%, specificity 79.2%, positive predictive value 94.3%, negative predictive value 59.4%).6 Mucin-producing neoplasms in the bile duct can produce a large amount of mucin, resulting in misdiagnosis of tumor location if only cholangiography is employed. Videocholangioscopy is very useful for accurate detection of the primary site of the tumor.9 Detailed observations make it possible not only to detect abnormal findings but also to accurately target biopsy sites by direct inspection (Fig. 26.4).
Bile duct neoplasms, in particular papillary growth type or mucin-producing neoplasms, often show a longitudinal tumor spreading from the primary bile duct lesions. Detailed imaging on enlarged images obtained by videocholangioscopy permits detection of tiny abnormal findings, regardless of benign or malignant nature.5,9 Furthermore, biopsy view videocholangioscopy using 3 Fr biopsy forceps provides good diagnostic ability.6 When the biliary stricture is too inelastic to allow passage by a videocholangioscope, a biliary dilating balloon catheter or temporary 10 Fr plastic stent placement can be effective.
Image-enhanced videocholangioscopy using NBI clearly displays fine biliary mucosal structures and capillary vessels (Fig. 26.5; Video 26.2) contributing to accurate biopsy and diagnosis of biliary tract diseases.
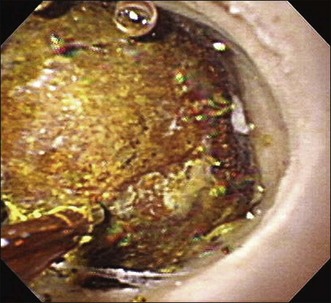
Although therapeutic videocholangioscopy is limited because of its small working channel, 1.9 to 3 Fr electrohydraulic lithotripsy (EHL) and laser lithotripsy using holmium YAG and FREDDY have been performed under direct videocholangioscopic visualization (Fig. 26.6; Video 26.3).
Adverse Events and Limitations
Videocholangioscopy, like fiberoptic cholangioscopy, can cause procedure-related adverse events such as cholangitis and pancreatitis. There are several limitations of the mother-baby videocholangioscopy system because of the endoscope fragility, expense of repair, and need for two skilled endoscopists. On NBI, cholangioscopy bile resembles blood, which can lead to poor images, and it is time consuming to clean the bile duct.4
Videocholangioscopy by the Direct Insertion System
Direct peroral fiberoptic cholangioscopy performed by the direct insertion technique was described by Urakami et al.11 3 decades ago using a standard upper gastrointestinal (GI) endoscope. However, this method has not become common because of the difficulty of direct insertion using a standard upper GI endoscope. In 2006 the first case series using ultraslim upper GI videoendoscopes was reported by Larghi and Waxman.12 Since then diagnostic and therapeutic direct peroral videocholangioscopy (DPVCS) have become increasingly common.13–23
Description of the Technique
DPVCS is usually performed using conventional ultraslim upper GI endoscopes (Table 26.3). However, since they have a 5- to 6-mm outer diameter, endoscopic sphincterotomy is mandatory. On occasion papillary dilation is added to facilitate scope passage across the papilla. It has four-way tip angulation and a 2-mm working channel.
Table 26.3 Direct Peroral Videocholangioscopy
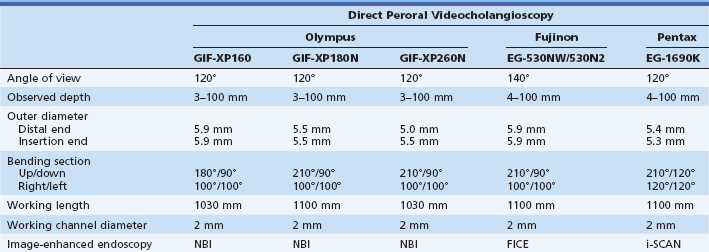
FICE, Flexible spectral imaging color enhancement; NBI, narrow band imaging.
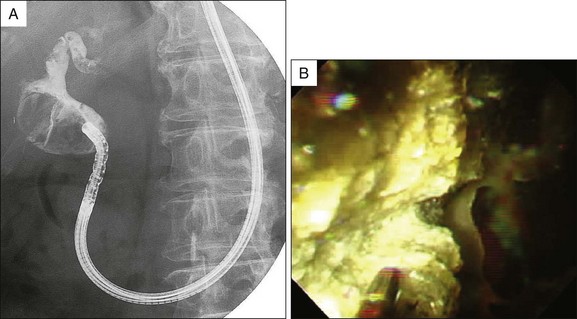
At the present time five approaches for direct bile duct access have been reported: (1) freehand insertion without any assisting devices, (2) wire-guided insertion, (3) balloon overtube–assisted insertion, (4) occluded duodenal balloon-assisted insertion, and (5) intraductal anchoring balloon-assisted insertion (Fig. 26.7).23 In general, freehand scope insertion usually appears difficult or impossible and wire-guided insertion or intraductal anchoring balloon-assisted insertion is frequently used. Several studies have described a success rate of intraductal balloon catheter-assisted insertion higher (95.2%, 20 of 21) than that of wire-guided insertion (45.5%, 5 of 11) or balloon overtube–assisted insertion (83.3%, 10 of 12).15,18
Technique: Diagnostic and Therapeutic
After reaching the bile duct segment of interest, DPVCS enables several diagnostic and therapeutic endoscopic procedures, such as observation or biopsy for diagnosis, or electrohydraulic lithotripsy (Fig. 26.8), laser lithotripsy, tumor ablation using argon plasma coagulation, photodynamic therapy, or biliary stenting using plastic or metallic stents through the 2-mm working channel. However, 2-mm accessories are not commonly available and are limited in number (Fig. 26.9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree