Jonathan S. Fisher, Jonathan R.T. Lakey, Christopher L. Marsh
Pancreas and Islet Transplantation
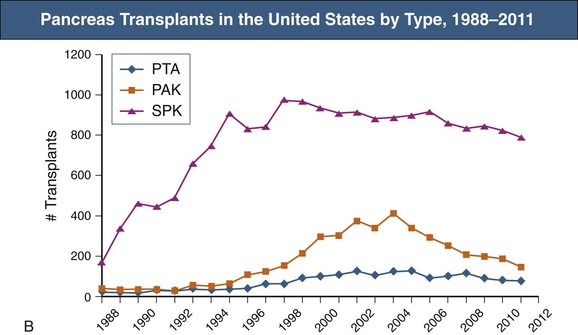
Pancreas transplants are performed for the amelioration of insulin-requiring diabetes. Initially, pancreas transplants were performed only in those diabetic patients with chronic kidney disease who needed kidney transplants; these patients underwent simultaneous pancreas-kidney transplantation (SPK). Today, pancreas transplantation alone (PTA) or pancreas transplantation after living or cadaveric renal transplantation (pancreas-after-kidney [PAK] transplant) is increasingly common. SPK accounted for 72% of all pancreas transplants in 2010 (Fig. 110-1). There were approximately 3400 people in the United States waiting for pancreas transplants in 2013.1 The 2011 Annual Report of the Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) reveals that the number of new registrations as well as of pancreata recovered and transplanted in the United States steadily increased from 1997 to 2004, but there has been some decline since then.2 Although there is less complete data for pancreas transplants outside the United States, the trends are similar with a decline in non-U.S. transplants performed since 2007. Several factors are thought to play a role. New insulin formulations and delivery systems in the form of insulin pumps have reduced the need for transplant in some patients. Selection criteria for potential recipients have become increasingly stringent as the average age of recipients has increased as well as the number of transplants being done in type 2 diabetic patients. Simultaneously, there has been a trend over time toward tighter donor criteria. Last, there have been recent downward trends in numbers of patients registered for pancreas transplants. New SPK registrations rose from 1412 in 1997 to a high of 2007 in 2000 and declined to 1671 in 2006. Some of this reduction may be the result of an increase in the number of patients receiving islet cell transplants. The relative roles of pancreas and islet cell transplantation remain controversial.
Patient Selection Criteria for Pancreas or Islet Transplantation
Indications for Transplantation
Indications for pancreas or islet transplantation include (1) insulin-dependent diabetes with associated diabetic complications (nephropathy, neuropathy, and retinopathy) and (2) diabetes with episodes of hypoglycemic unawareness.
A number of factors influence the choice between pancreas transplant and islet transplant. Because giving sufficient islets remains a limiting factor, islet transplants are more appropriate for patients with smaller insulin requirements, typically slender women. Larger patients (usually with higher insulin requirements) are more reliably served with whole-organ pancreas transplants. Islet transplantation is performed with a radiographic procedure and therefore is better suited for patients who cannot tolerate the surgical stress of whole-organ transplantation, such as older patients with severe coronary heart disease.
Pancreas or islet transplantations have not routinely been performed for type 2 diabetes mellitus because the primary defect in type 2 diabetes is insulin resistance, not insulin deficiency. There may be a role for pancreas transplantation in type 2 diabetic patients who develop islet failure after years of insulin resistance or those who have deficiencies in beta cell function such as maturity diabetes onset of the young (MODY, types 1 to 6) or mutations of the insulin receptor gene (e.g., type A insulin resistance or Rabson-Mendenhall syndrome).3 A recent review of the United Network for Organ Sharing (UNOS) registry from 2000 to 2007 revealed that after adjustment for a variety of factors (e.g., age, race, weight, time on dialysis, cardiovascular disease), there were no differences in risk for death or kidney or pancreas failure according to diabetes classification.4 The analysis suggested that insulin-requiring patients who have type 2 diabetes, are younger than age 50 years, and have a body mass index (BMI) below 30 kg/m2 will become insulin independent with a pancreas transplant. The proportion of pancreas transplants that are performed in type 2 diabetics increased from 2% in 1995 to 7% in 2010.5
Most pancreas organs for transplantation come from deceased donors. However, one approach to increasing the donor supply has involved living donor laparoscopic distal pancreatectomy with or without simultaneous laparoscopic nephrectomy.6
Should living kidney donor recipients be offered a PAK transplant? An analysis of the UNOS database revealed no difference in survival of SPK recipients and living kidney donor recipients at up to 8 years of follow-up.7 However, a European study showed an improved 10-year patient survival (83% in SPK versus 70% in kidney transplants alone) and noted a significantly lower progression of macrovascular disease (cerebrovascular, coronary, and peripheral vascular) with SPK transplants.8 A review of the UNOS registry from 1997 to 2007 divided recipients with type 1 diabetes who received a living donor kidney transplant into two groups: (1) those who subsequently received a PAK transplant within a year of the kidney transplant (n = 1026) and (2) those who did not (n = 3528). At the end of the eighth year, the patients who subsequently received a PAK transplant had superior patient (85% versus 75%) and kidney graft survival (75% versus 62%).9 Therefore the two-stage approach of PAK after a living donor kidney transplant should perhaps be offered to patients who have a living kidney donor and in whom the cardiovascular risk of the extended procedure required for SPK is considered too great.
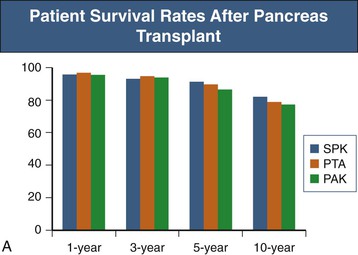
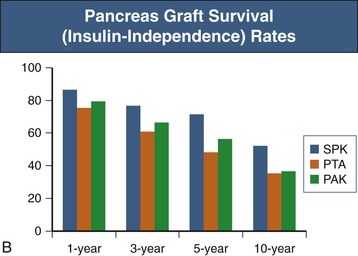
The role of PTA in those with preserved renal function has recently been questioned. A single-center study of 131 PTA recipients revealed that PTA is an independent risk factor for the development of renal failure, presumably because of the nephrotoxicity of long-term immunosuppression.10 An analysis of the UNOS database demonstrated worse survival for those with diabetes and preserved kidney function receiving a PTA than for those who remained on the waiting list and received conventional therapy.11 The recent SRTR data reveal 1-year patient survival rates similar for PAK, SPK, and PTA recipients (ranging from 95% to 97%). However, the 10-year patient survival rate was lowest for PAK recipients at 77% and similar for SPK and PTA recipients with rates of 82% and 71%. SPK resulted in the best pancreas graft survival rates, 86% at 1 year and 52% at 10 years. Graft survival rates for PAK and PTA recipients were slightly worse and similar to each other, with 1-year rates of 79% and 75%, respectively, and 10-year rates of 37% and 35%, respectively (Fig. 110-2).1 Further close study is warranted, keeping in mind that a prospective, randomized trial of pancreas transplantation versus conservative therapy is not practical.
Medical Evaluation
The medical evaluation of the prospective pancreas transplant candidate is similar to that of the kidney-only recipient (see Chapter 102), although the cardiac workup is more extensive. The best candidates for transplantation are younger than 50 years and have a limited number of major complications of diabetes, such as hypoglycemic unawareness or diabetic neuropathy. Additional complications, such as vascular disease, orthostatic hypotension, and severe gastroparesis, put patients at higher risk of post-transplantation complications, but none of these factors by themselves exclude a patient from transplantation. Cardiovascular status is the primary deciding factor for transplantation eligibility because the surgery, infections, risk of thrombotic complications, and, until recently, rejection are more severe in the pancreas transplant recipient, demanding that the cardiovascular system be able to withstand multiple prolonged, hemodynamically stressful events. All patients require noninvasive cardiac stress evaluation because of the limited exercise capabilities of many patients. Cardiac catheterization is performed on the basis of the results of noninvasive testing or performed first for high-risk patients, typically those older than 45 years, those with diabetes duration of more than 25 years, smokers of more than 5 pack-years, and those with an abnormal electrocardiogram. Peripheral vascular disease is evaluated by clinical examination and by arterial duplex ultrasound. Patients with limb-threatening ischemia are typically poor pancreas transplant candidates. The medical evaluation for islet transplantation is similar to that for pancreas transplantation, but exclusion criteria are fewer because of lower surgical and inflammatory risks.
The last criteria for transplantation are that the donor and recipient match for ABO blood group and that the recipient sera are crossmatch negative against donor T cells by either the standard antiglobulin or flow cytometry crossmatch.
Pancreas Transplantation
Patient and Graft Survival
Pancreas graft survival rates have increased as a result of improved surgical techniques, donor selection criteria, and improvement in the composition of the preservation fluid, as well as more effective immunosuppressive regimens, despite an increasing proportion of high-risk patients.
There has been a trend toward tighter donor selection criteria. The ideal donor can be defined as 10 to 40 years of age, with a BMI less than 27.5 kg/m2 and a cause of brain death that is other than cerebrovascular.12 The UNOS database shows that over the past 5 years more than 60% of donors have had a cold ischemic time of less than 12 hours.2
The most commonly used cold storage solution is the University of Wisconsin (UW) solution, which has increased early pancreas graft function and reduced the occurrence of preservation pancreatitis. A second cold storage solution, histidine-tryptophan-ketoglutarate (HTK), offers the advantages of better tissue perfusion because of lower viscosity, less reperfusion hyperkalemia, and significantly lower cost. However, studies have suggested a higher incidence of graft pancreatitis and an increased rate of graft loss with HTK, particularly with longer cold ischemia.13,14 Approaches using either a two-layer storage method with UW or an HTK solution and a second layer of highly oxygenated perfluorocarbon or the attachment of the pancreas to a low-pressure pulsatile perfusion system are being investigated.
The current gold standard immunosuppression for pancreas transplants is antibody induction therapy, tacrolimus, mycophenolate mofetil (MMF), and corticosteroids; this has led to a 40% decrease in incidence of rejection and an increase in 1-year graft survival to more than 90%. UNOS registry data show that human leukocyte antigen (HLA) matching has no effect on the outcome in SPK, whereas for PAK and PTA, a beneficial effect is seen by matching at the A and B loci. The reported 1-, 3-, and 5-year patient and graft survival rates with use of these techniques and criteria are shown in Figure 110-2.1
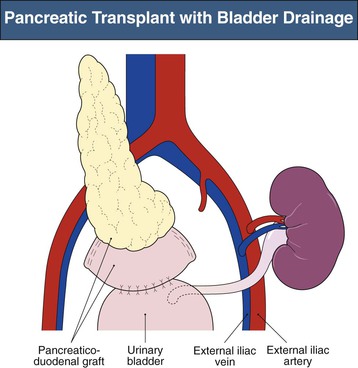
Enteric Drainage of the Pancreas Transplant
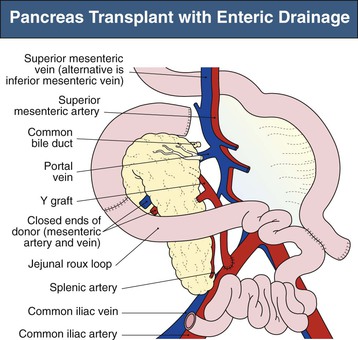
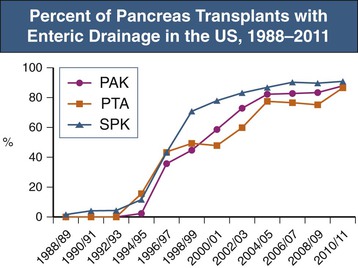
Current practice is to transplant the whole pancreas with a cuff of duodenum, which preserves the blood supply of the head of the pancreas and provides a means to drain exocrine secretions into either the small bowel (enteric drainage) or the bladder (Figs. 110-3 and 110-4).1 From 2004 to 2008, the majority of the pancreas transplants in the United States were enterically drained (Fig. 110-5).1 The graft may be placed in the right iliac fossa like a kidney, intraperitoneally, and vascularized from the common iliac vessels so that the secreted insulin enters the systemic circulation. The right side is preferred to the left because the vessels are somewhat more shallow on the right side and consequently the anastomoses are easier to perform. However, an alternative is to construct the venous anastomosis to the superior mesenteric vein, allowing more physiologic insulin output through the portal circulation. Currently, 15% to 20% of enterically drained transplants use portal drainage. In either approach, an interposition graft from the donor (typically a Y graft containing common iliac with external and internal branches) is used to provide inflow from the recipient common iliac artery to the graft superior mesenteric and splenic arteries. Some surgeons have advocated reconnection of the graft gastroduodenal artery if the pancreaticoduodenal arcades are not intact. Pancreas transplants with venous outflow to the superior mesenteric vein can be placed either anterior to the small bowel mesentery or in a retroperitoneal position behind the ascending colon, where the superior mesenteric vein is reached from the side. Surgical outcomes have not differed whether the venous drainage is systemic or portal. Although portal drainage is considered more physiologic and avoids hyperinsulinemia, these apparent benefits are not well confirmed.
Immunosuppression
Most centers use antibody induction therapy during the first 1 to 2 weeks after transplantation. OKT3 has largely been replaced by antithymocyte globulin (ATG).15 Other centers use an interleukin-2 receptor antagonist (basiliximab) or, most recently, an anti-CD25 antibody (alemtuzumab).16,17 Antibody-mediated rejection may be more common than acute cellular rejection in patients receiving alemtuzumab.18 Most centers use triple-drug maintenance immunosuppression with tacrolimus (or less commonly cyclosporine), MMF (or rarely azathioprine), and corticosteroids. There is increasing evidence of equivalent success with rapid corticosteroid elimination or corticosteroid avoidance protocols. Some centers replace the calcineurin inhibitor or MMF with sirolimus. With the more profound immunosuppressive induction produced by the newer antibodies (particularly alemtuzumab), there have been reports of immunosuppression protocols limited to a depleting antibody and a single additional agent.17,19,20 Fortunately, cytomegalovirus (CMV) infection rates appear to be lower in corticosteroid-free regimens,17 although there has been some increase in CMV infection in those receiving depleting antibody therapies.16
Graft Monitoring
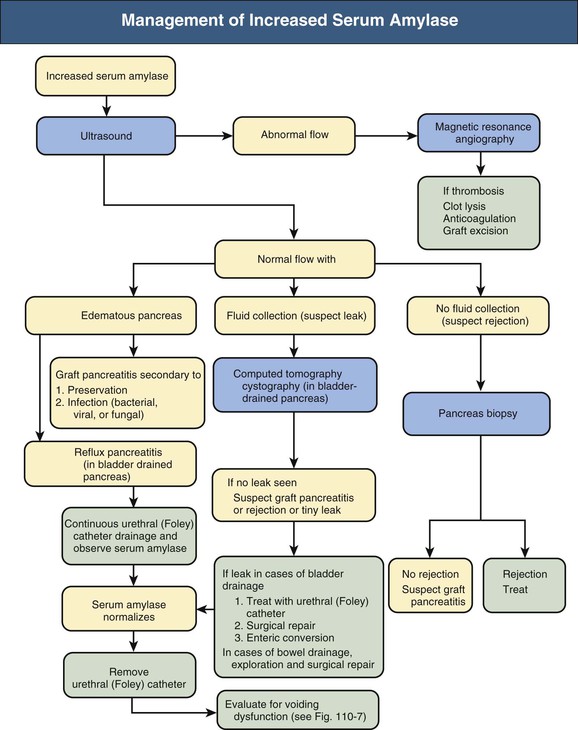
The causes of pancreas graft dysfunction and the evaluation process are shown in Figure 110-6, Box 110-1, and Table 110-1.
Table 110-1
Evaluation of pancreas graft dysfunction.
| Evaluation of Pancreas Graft Dysfunction | |
| Assessment | Tests |
| Laboratory tests | Serum amylase, blood glucose human anodal trypsinogen, cyclosporine or tacrolimus levels, C peptide In bladder-drained cases, urinary amylase and urine culture |
| Doppler ultrasound | Pancreatic blood flow, peripancreatic fluid collection, pancreatic ductal dilation In bladder-drained cases, evidence of bladder outlet obstruction |
| Computed tomography with or without cystography | Looking for leak and collections; this is performed by cystography in bladder-drained cases |
During the immediate perioperative phase, intravenous insulin is used to decrease the stress on the transplanted pancreas by maintaining serum glucose concentration around 100 to 120 mg/dl (5.5 to 6.6 mmol/l). Serum glucose values are not an early marker of pancreas dysfunction; elevations are observed only after significant parenchymal pancreatic damage has occurred. With bladder drainage, urinary amylase excretion is measured on 12-hour collections and reported as units per hour. During the first 1 to 2 weeks after transplantation, serum amylase may be elevated as a result of pancreatic preservation injury. Trends are followed rather than absolute values, taking into consideration factors such as length of cold ischemic time and degree of organ edema. Stable serum levels are usually attained within 2 weeks after transplantation. Rising serum amylase or lipase concentration indicate possible graft injury, because both are moderately sensitive markers of pancreas rejection. However, other conventional causes of pancreatitis can still occur. Elevated fasting glucose and 2-hour postprandial glucose levels are relatively late indicators and only indicate dysfunction without revealing cause.
Ultrasound examination of the pancreas transplant is performed frequently in the early post-transplantation period to rule out vascular thrombosis. If the pancreas cannot be well visualized on ultrasound scan, magnetic resonance imaging and angiography may be informative. Any inflammatory state can also yield images of an edematous pancreas; however, other than for vascular thrombosis, imaging generally cannot reveal the cause of the dysfunction.
Biopsy of the pancreas transplant remains the gold standard for diagnosis of acute or chronic rejection. A biopsy may be performed at certain time points by protocol or at times of graft dysfunction to identify rejection or other causes of pancreatic injury before irreversible tissue damage has occurred. The easiest approach is a percutaneous biopsy with ultrasound or computed tomography guidance. The most frequent complication of percutaneous biopsy is a perigraft hematoma or transient hematuria, but rarely seen are pancreatitis, arteriovenous fistula, abdominal hemorrhage, bowel perforation requiring exploration, and even graft loss. Because of the risks associated with biopsy, if the clinical picture is consistent with a mild case of rejection, patients may be treated for rejection without biopsy confirmation.
Treatment of pancreas rejection is similar to that of kidney rejection and generally involves pulse intravenous corticosteroids or antilymphocyte antibodies (see Chapter 104).
Treatment response is achieved by following the return of serum amylase and lipase to baseline values. Imaging can be used to show resolution of edema and inflammation. Repeated biopsy, usually after a 2-week interval, is required to show resolution of more moderate or severe rejections and to look for histologic signs of the development of chronic rejection.
Patients with isolated pancreas rejection have an increased risk for kidney graft loss, supporting the concordance of acute rejection in the majority of patients.21,22 In SPK or PAK patients, when biopsy of the pancreas cannot be performed safely, a kidney transplant biopsy may be used as a surrogate indicator. This must be done, however, in conjunction with serum tests because synchronous rejection occurs only 70% to 80% of the time.23
Antimicrobial Prophylaxis
Antimicrobial prophylaxis is much like that for a kidney transplant alone. Trimethoprim-sulfamethoxazole is prescribed for the prevention of urinary tract infections and Pneumocystis infection. Oral clotrimazole or nystatin is used for the prevention of oral candidiasis; some centers use fluconazole for prophylaxis of Candida urinary tract infections and intra-abdominal fungal or yeast infections. Oral acyclovir is given to patients with a history of herpes simplex infection and to patients who are CMV negative and receive CMV-negative donor organs. Otherwise, valganciclovir or ganciclovir is given for 3 months after transplantation to all patients who are CMV positive. Patients who are CMV negative who receive CMV-positive organs are often treated for 6 months. Patients treated for rejection are typically returned to any discontinued anti-infectious prophylaxis for 1 to 3 months after rejection therapy.
Metabolic Monitoring
In addition to monitoring of the serum and urinary concentrations of amylase and the serum concentration of lipase, serum creatinine, potassium, magnesium, and bicarbonate levels must be monitored. Magnesium wasting is common with calcineurin inhibitors and frequently requires oral supplementation.
Surgical Complications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree