Fig. 21.1
CT scan demonstrating a port-site hernia
The Veress needle is an alternative to the open technique and can be used alone or in conjunction with an optical view technique. Traditionally, Veress needles are 14 gauge in size and are spring-loaded so that when resistance is met, the blunt end retracts into the needlepoint and tissues such as fascia and peritoneum can be traversed without significant trauma. The operator must feel the needle insert into the peritoneal cavity and discontinue advancing once the requisite number of “pops” is felt and no further resistance is encountered. When centered on the midline, the operator will experience two points of resistance (the midline fascia and the peritoneum), with the latter causing the spring mechanism to “pop” as resistance is encountered and the needle abruptly discontinued (corresponding to the needle traversing the peritoneum and entering the peritoneal cavity). Confirmation of safe access is determined by water drop test in which saline is introduced into the end of the Veress needle, which if correctly placed will passively flow into the abdomen due to the lower intraperitoneal pressure relative to the atmosphere. I find this test to be finicky and prefer to simply connect the insufflation tubing set on a flow rate of 3 L/m and assess my opening pressures as determined by the insufflator. The initial opening pressure should be low single digit or zero mmHg but will correlate with the patient’s body mass index [8]. Anything higher in a patient who is not morbidly obese indicates that the needle is not in the correct location and insufflation should be terminated immediately and the needle repositioned or an alternative method of initial access should be pursued. Veress needles can be used in the midline, but for re-operative surgery (in which the midline has been used), an off-midline technique can be employed. Proponents of this technique recommend the left upper quadrant (Palmer’s point) as the preferred site [9] as there is relatively little that can be injured here. In contrast, the liver edge often obscures the right upper quadrant, while the lower quadrants risk bowel and vascular injury and are less desirable locations. Many surgeons will want to elevate the abdominal wall when using this technique, but this may make it more difficult by pulling up on the skin and creating a long distance between the underlying fascia and the skin entry site. Many bariatric surgeons recommend not elevating the skin and simply inserting the needle. The same haptic feedback is achieved irrespective of skin elevation, and it won’t be necessary to insert the full length of the needle to gain access into the peritoneum in someone with a lot of subcutaneous fat.
Injuries associated with the Veress needle entry encompass the entire spectrum of bad things that can occur with initial trocar injury—vascular, bowel, bladder, solid organ, and air embolism have all been described [2]. The key to success in using a Veress needle is experience and discipline. Multiple passes should never be needed to gain access, and the entire length of the needle (usually 12–15 cm) should rarely be needed to reach the peritoneal cavity.
The optical view technique or direct trocar insertion is newer than either the Veress or open Hasson techniques and relies on the clear plastic material of most modern trocars. A zero-degree laparoscope should always be used inside the trocar and the focus should be adjusted so that it is just beyond the tip of the trocar. Elevating the abdominal wall is not necessary and can be detrimental in an obese patient, as the trocar length will not traverse the abdominal fat. The layers of the abdominal wall can be visualized as the bladeless obturator passes through (Video 21.1). When used in conjunction with the Veress needle, the operator will have no difficulty identifying the peritoneal cavity as it will be insufflated with gas (Video 21.1). If the optical view technique is to be used without establishing pneumoperitoneum beforehand, the surgeon must be experienced in identifying the layers of the abdominal wall as seen through the trocar [10]. Otherwise, even experienced surgeons who do not routinely use this technique can find themselves below the omentum or pre-peritoneal (Video 21.2).
Enterotomy, Serosal, and Thermal Injuries
Key Concept: Enterotomies can and will occur during laparoscopy. Initial trocar entry is a risk as is lysing adhesions in a re-operative abdomen. Immediate control and repair or resection is necessary to limit contamination.
Enterotomies can and will occur during the conduct of laparoscopic abdominal surgery. There is a risk of bowel injury associated with the initial trocar access which has been estimated between 0.5 and 0.7 % [11, 12]. Re-operative surgery also increases the risk of an enterotomy, and during re-operative colectomies, the risk is estimated to be less than 1 %; however, this is still significantly higher than the incidence when operating on a virgin abdomen [13]. When dealing with inter-loop small bowel and pelvic adhesions, the use of sharp dissection technique is preferred to avoid injury resulting from energy and heat (Video 21.3). Inadvertent bowel or serosal injuries can often be repaired if a result of sharp dissection when the true extent of the injury can be determined. It is imperative that once an enterotomy is made, it is identified and repaired immediately (Video 21.4). This will avoid any unnecessary contamination and spillage as well as the risk of not being able to find it later in the procedure. If the surgeon is not comfortable evaluating and closing the enterotomy laparoscopically, a small abdominal incision can be made and it can be repaired extracorporeally. Prior to exteriorization, the bowel should be tagged to facilitate identification of the injury.
Intestinal injury can also occur off-camera, and great care should be taken to avoid forcing an instrument into the operative field as it may be caught up in the small bowel. This is particularly true when the patient is positioned in either extremes of Trendelenburg or airplaned in either direction. In addition, when using energy sources, heat is generated on the blade of the instrument that can cause small bowel injury both on and off the camera as the instrument is withdrawn. Care should be taken when removing any instrument that is potentially hot, and it is good practice to allow the instrument to cool prior to removing.
A relatively common scenario during laparoscopy is an inadvertent serosal or thermal injury. It is our practice to repair serosal injuries irrespective of laparoscopy using a 3-0 Vicryl Lembert suture. It is important that this be done as soon as they are recognized, as they can be difficult to relocate after even a few minutes (Video 21.4). This is particularly true during a laparoscopic case where inspecting the entire bowel can be much more labor intensive than in open surgery. The utility of oversewing serosal injuries is not well studied, but animal models have failed to identify any benefit [14]. It is likely that very superficial injuries occur frequently and go unrepaired without detriment to the patient, but in the absence of demonstrable harm, we suggest repairing recognized serosal injuries for fear of delayed intestinal perforation and leaks. It is also important to recognize thermal injuries (Fig. 21.2), which can occur during use of electrosurgical devices and bipolar and ultrasonic energy devices. It is estimated that such injuries occur between 0.6 and 3 times per 1,000 cases [15]. Electrothermal injury may result from direct application, insulation failure, direct coupling, and capacitive coupling. Direct application is probably the most common and easiest to recognize. Once again, it is important to immediately evaluate the injury and decide if it necessitates repair. Finding the injury at a later time will prove difficult irrespective of laparoscopy. The decision to oversew these injuries will depend on the operator’s judgment as to whether the bowel wall integrity has been compromised. Coagulation burns are deeper than those caused by a blended or cutting current [16], and injuries that blanch white have usually gotten hot enough to cause protein denaturation, but may not result in full-thickness injury. As a rule, if the injury is a result of a very short burst of energy and there is minimal tissue change, no further intervention is required.
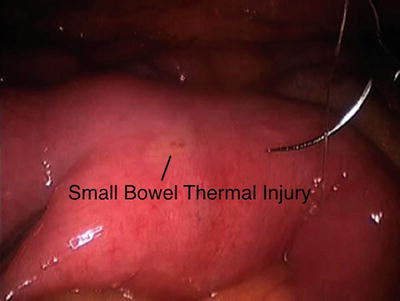

Fig. 21.2
A thermal injury is identified on the small bowel antimesenteric surface. Notice that there is a degree of blanching with some very small visible burn formation. This may not have created any problems but it is safer to be proactive when dealing with these types of injuries
Bleeding: Intra-abdominal and Pelvic
Key Concept: There are a variety of options for ligating blood vessels intracorporeally, and the surgeon should master one of these instruments and then have a plan for when they fail. All methods for ligating blood vessels are capable of failing.
The development of reliable energy sources to ligate major vascular pedicles has been a critical step in moving laparoscopic colorectal surgery toward the mainstream. Surgeons now have a reliable method of dividing large (up to 7 mm) vessels and with this the opportunity to encounter major bleeding, both during and following surgery which may challenge the laparoscopist more so than during open surgery. When major bleeding occurs, the laparoscopic surgeon must rely not only on their skill but also those of a talented assistant. Certainly, silk and Vicryl ties can fall off of major arteries and veins, but controlling bleeding during an open surgery is generally less difficult when compared to laparoscopy, and the operator will need to have a skill set and a plan of action when major bleeding occurs during the conduct of a laparoscopic case.
The current choices for vascular ligation include laparoscopic staplers, clip appliers, bipolar energy, and radiofrequency (ultrasonic) shears or some variation of these themes. In general, the bipolar products have similar performance characteristics and are approved for 7 mm vessel ligation [17]. Many authors have favorably compared ultrasonic devices with bipolar energy, although the vessel indication is smaller (5 mm) and may result in longer operative time and greater blood loss when compared to bipolar devices [18–20].
The use of monopolar electrocautery is appropriate for dissecting in the avascular anatomic planes, but will not be effective in dealing with any vessel of significant size. The use of clips on mesentery and vascular pedicles has largely been abandoned, as they are inferior to the other readily available options [19, 21, 22].
It is preferable to be facile with one technology, as repetitive use will result in less bleeding from erroneous application. The cost-effectiveness of staplers vs. energy devices has been evaluated [20], and it is my practice to use staplers to divide the pedicle only if I plan to divide the bowel intracorporeally—a situation in which I would not open any energy device. All energy devices generate heat and have some degree of thermal spread. It is important to be mindful of this when exchanging instruments or when dividing tissue near important structures that are being preserved. It is also critical to avoid tension when ligating major vessels, as this can result in inadequate tissue sealing and bleeding. Arteries that are heavily calcified may not seal with energy due to the inability of the proteins to coagulate and an alternate approach may be preferable in this situation. Fortunately when energy devices fail, they tend to do so immediately and delayed bleeding and take backs for vessels that were clearly sealed at the initial operation are rare [23].
When bleeding does occur, the most important initial step is to gain proximal control—an attempt to clip or ligate a bleeding mesenteric or named arterial vessel without first controlling the source will be unlikely to work and can result in injury to important structures (Video 21.5). A Maryland grasper works well for this purpose and it is a good instrument to have on the Mayo stand at all times. It is good practice to abandon the technique that resulted in the bleeding and to proceed directly to an ENDOLOOP ® (3-0 PDS™ II works well in this situation, Ethicon Endo-Surgery, Cincinnati, OH). Reattempting to seal a vessel that is bleeding using energy may work, but if the proximal side is short and near the mesenteric root, the stakes for failure can be very high. This is particularly true when dealing with bleeding venous structures, as they can be very unforgiving. It is my practice to always divide the inferior mesenteric vein a few centimeters distal from the duodenum in the event that if it does bleed, it can be controlled. If it is ligated very short, it may retract behind the pancreas and can rarely be salvaged laparoscopically.
When bleeding is somewhat diffuse and the source is difficult to identify, the operator has a few options—the laparoscopic suction irrigator can be difficult to use for this purpose, as it will often become occluded with tissue. Vaginal packing works well in this situation—cut to 15–20 cm strips, it can be introduced through a 12 mm trocar and act as a sponge and can be used in concert with the suction irrigator (Video 21.6). Additionally, nasal packing strips are a similar size and can be easily placed down a standard size trocar. Care should be taken to remove either right away. A sponge can also be used but will be more likely to fray and may not retain its radiopaque strip when cut. A laparotomy pad can be introduced through a hand port without much difficulty and is also a nice way to clear the field. It is imperative to remain calm during bleeding that is difficult to control. It is appropriate to attempt laparoscopic control because the time required to turn the lights on, get the nursing staff oriented, open the patient, and isolate the bleeding will typically result in more blood loss. Therefore, compress the site of bleeding with a sponge, and if it is not possible to isolate, then maintain the pressure to stem the bleeding during conversion.
Pelvic bleeding can be a source of considerable hemorrhage in both open and laparoscopic surgery. Control of presacral bleeding can be accomplished laparoscopically [24] through a variety of techniques (Video 21.7) including a welding technique using the rectus muscle of epiploic fat. The use of bovine pericardium has also been described applied to the bleeding site with a spiral tacker. The best strategy is to stay in the correct planes posteriorly and use an energy device laterally to divide the lateral stalks and peritoneum. This will keep the field dry and maintain the critical exposure, which can be difficult in a narrow pelvis.
On rare occasions when pulsatile bleeding strikes the camera, the operative field will be totally obscured—creating a situation that is particularly unnerving. It is important to determine the significance of the bleeding (omental vessel vs. IMA) and to deal with it as quickly as possible. Typically the camera operator is the least experienced surgeon or student involved in the case, and the senior surgeon must quickly take control of the situation. There is no point in proceeding until the visualization of the field can be restored; therefore, the first priority is to clear the lens by removing the laparoscope. Blood in the trocar will frustrate any attempts at good visualization, and if it cannot be cleared quickly, therefore, an alternate trocar should be chosen for the camera as long as it provides good exposure to the bleeding vessel. Alternatively, a 5 mm trocar can be upsized to accommodate a 10 mm laparoscope, which will be less temperamental in the face of blood and debris. Once the operative view has been restored, an assessment of the bleeding can be made and dealt with appropriately. When necessary, an additional 5 mm trocar can be inserted to provide a point of entry for additional instruments or an ENDOLOOP ® . Never allow the lack of an additional 5 mm or 10 mm trocar to result in a conversion, advice that is often lost in the stress of the situation.
Anastomotic Leak
Key Concept: The most important intraoperative predictors of a healthy anastomosis are adequate blood supply and absence of tension. In the event that either of these is not achieved, the laparoscopic surgeon will need to decide if converting the case will offer a better chance of success. A technically perfect anastomosis requires intimate knowledge of the tools being used.
There are innumerable studies looking at the risk factors and strategies to prevent anastomotic complications with some general themes that are consistent. Prevention of leak starts with a meticulous surgical technique, and surgeons can have the biggest impact on prevention of anastomotic complications by ensuring that the blood supply to the anastomosis is intact and that there is no tension across the anastomosis. While there is little in the way of data to support the latter assertion, there is a host of newer data correlating the oxygen tension in the mucosa of the bowel with rates of anastomotic leak. Testing the effect of tension across anastomosis has been done in animal models with demonstration of decrease of mucosal blood flow in the face of increasing tension. The presence of mechanical forces attempting to disrupt anastomosis does not require a study to demonstrate poor outcomes. The surgeon should do whatever is necessary to make sure that the bowel that is being joined together does so easily and without tension. The risk of leak for a right colon anastomosis should be very low, as blood supply and tension should never be an issue. Care should be taken to avoid the “180-degree twist” that is unfortunately easier to do than believed with side-to-side anastomosis and can result in kinking in the blood supply. For a left colectomy, both blood supply and tension can be problematic. The surgeon’s decision to ligate the inferior mesenteric artery at its origin will have an impact on the blood supply to the subsequent conduit. If the descending colon or transverse colon is to be used as the conduit, the impact is mitigated. With the sigmoid colon, the marginal blood supply off of the middle colic may not be adequate to perfuse such a long conduit [25, 26]. If it is necessary to use the sigmoid colon as part of the colorectal or coloanal anastomosis, the left colic artery should be preserved [27, 28]. Length can be achieved by completely mobilizing the attachments of the left colon to the retroperitoneum and flexure. The other critical aspect to obtaining adequate bowel length is mobilizing the mesentery, which will tether the left colon into the abdomen unless it is freed. To gain additional length, the inferior mesenteric vein (IMV) must be ligated adjacent to the IMA and a second time at the inferior border of the pancreas just lateral to the ligament of Treitz (Fig. 21.3). Ligating the vein twice while carefully preserving the marginal artery at the splenic flexure will add several centimeters to the length of the conduit while preserving arterial blood supply. A common error in an effort to gain length is to divide the colonic mesentery up toward the splenic flexure of the colon, with the end result cutting off the blood supply to the distal conduit, which is now based on the middle colic artery. If the marginal blood supply is compromised due to inadvertent injury while mobilizing the flexure or wandering too close the mesenteric border during ligation of the mesentery, the conduit will become ischemic and very likely unusable. As a general rule, if the cut edge of the mesentery traversing the pelvic brim is too tight to allow a finger (or a laparoscopic 5 mm grasper) to easily slip underneath (Fig. 21.4), the anastomosis is at risk since the blood supply is under tension—even if the bowel ends appear to approximate easily without tension. Every effort should then be made to lengthen the mesentery, even if this has already been attempted, as often reassessment will identify a small adhesion to release. In general, if the mesentery is lax, there is likely no tension at the anastomosis. In those cases where only a few centimeters would allow less tension on the anastomosis, distal mobilization of the rectum to elevate it out of the pelvic hollow can also be a useful maneuver.
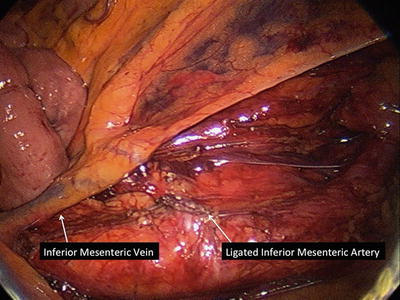
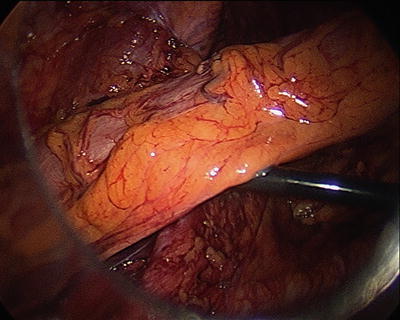

Fig. 21.3
The inferior mesenteric vein terminate in the splenic vein and will not be paired with the artery close to this location which can tether the conduit making it difficult for the anastomosis to be created without tension. The vein can be located in this location near the ligament of Treitz and ligated with clips, staplers, or energy

Fig. 21.4
In this figure the conduit can be seen traversing the pelvic inlet and a grasper can easily slip below without tension
There are unfortunately times when despite fully mobilizing both the mesentery and the left colon, the conduit simply won’t reach the pelvis. This can be due to a number of factors but is usually associated with a short fatty mesentery. You then find yourself in a situation where gaining length means dividing more mesentery (often the transverse mesocolon), which can result in further ischemia to the conduit and need for more length. There are limited options when this occurs—perform a total colectomy and an ileorectal anastomosis or rotate the right colon 180 degrees around the ileocolic pedicle in an effort to preserve the ileocecal valve. The latter option referred to as the Deloyers procedure [29] has proven successful, although a comparison to an ileorectal anastomosis has not been reported (Fig. 21.5a,b). Presumably the simplicity of an ileorectal anastomosis in cases where the entire rectum is preserved would outweigh the benefits of preserving the right colon. However, if part of the rectum has been resected, then the functional results of an ileorectal anastomosis are likely to be poor, and the Deloyers procedure would obviate the need for a permanent ostomy. The blood supply for this procedure is dependent on the ileocolic artery, and all mesenteric attachments of the ascending colon should be divided, being careful to preserve the marginal vessel adjacent to the right colon. The colonic segment is then rotated clockwise and anastomosed to the rectum. A window in the ileal mesentery has also been described, but is not necessary, as the colon will be situated anterior to the small bowel with this maneuver. Manceau et al. described their experience with this procedure in 48 consecutive cases [30] with a median follow-up of 26 months. There were no anastomotic leaks in this series, although 65 % of the patients had a temporary diverting ileostomy.
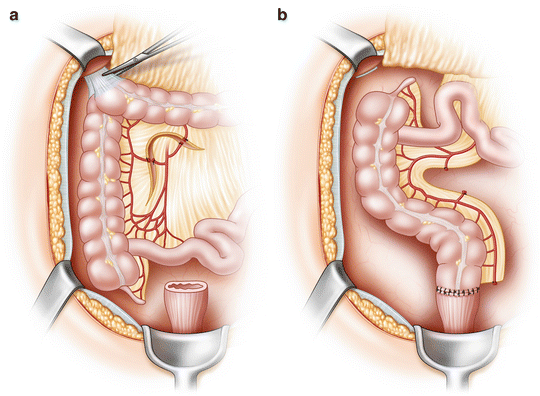

Fig. 21.5




Deloyers procedure. (a) The attachments to the right colon are taken down and the vessels are divided as shown. (b) The right colon is rotated 180 degrees around the ileocolic pedicle in an effort to preserve the ileocecal valve, and anastomosis is performed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








