Fig. 20.1
Diagram illustrating the fascial planes. The rectum is connected to the sacrum at the level of S4 by the rectosacral fascia called Waldeyer’s fascia
The dentate line signifies the anorectal junction. The top of the anal canal can be identified on palpation of a muscular complex consisting of the internal and external sphincters as well as the puborectalis sling. Distance of the tumor from this important landmark determines the ability to preserve the sphincters during resection. The tumor must be proximal enough from the anorectal ring to ensure a 1–2 cm distal margin. If this margin cannot be achieved, or if sphincter function would be compromised, then an APR should be performed.
The vascular supply of the upper and middle rectum is provided by the superior rectal artery as it branches off the inferior mesenteric artery. The inferior rectal artery branches off the internal pudendal arteries originating posterolaterally and supply the anal sphincters and anal epithelium. Although the lateral rectal stalks are thought to contain the middle rectal arteries, this is true only 22 % of the time [8]. These stalks predominantly contain nerves. The superior rectal vein drains the superior and middle rectum, emptying into the inferior mesenteric vein. The middle rectal vein drains the distal rectum and proximal anal canal via the internal iliac veins. Lastly, the inferior rectal veins drain the distal anal canal via the pudendal veins.
Lymphatic drainage is achieved within the mesorectum and follows the vascular supply (Fig. 20.2). Tumor cells can spread cranially along the superior rectal vessels necessitating a high ligation of the vascular supply at its origin for adequate staging and local control. The hypothesized amount of distal spread has influenced the recommended distal margin. Recently, distal spread has been shown to rarely extend beyond 1 cm allowing decreased distal margins for adequate control of disease [9–16] and improved sphincter preservation. Lymph traveling from the lower rectum can drain along any of the rectal arteries, emptying into the iliac vessels and ultimately the periaortic nodes.
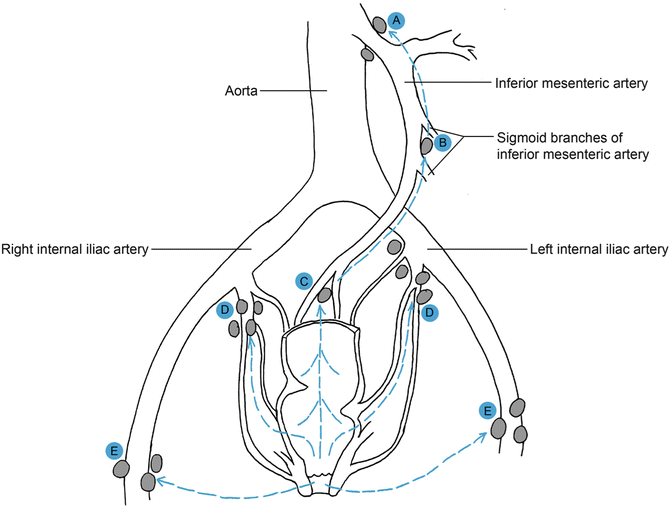

Fig. 20.2
Lymphatic drainage of the rectum and anus. The nodes at the origin of (a) inferior mesenteric artery and (b) the sigmoid branches. The (c) sacral, (d) internal iliac, and (e) inguinal nodes are represented
Innervation within the pelvis consists of paired sympathetic (hypogastric) nerves, sacral parasympathetic nerves, and inferior hypogastric nerves. The sympathetic nerves originate from L1 to L3 and descend as the hypogastric nerves along the sacrum. The parasympathetic innervation (nervi erigentes) originate from S2 to S4, joining the hypogastric nerves anterior and lateral to the rectum. These nerves form the pelvic plexus as well as the periprostatic plexus. Fibers from these sympathetic and parasympathetic plexi innervate bladder, ureter, prostate, seminal vesicles, membranous urethra, and corpora cavernosa. Injury to these nerves during dissection can lead to side effects such as impotence, retrograde ejaculation, bladder dysfuction, urinary retention, and loss of normal defecatory mechanisms.
Indications for Operation
Low anterior resection (LAR) is defined as resection of the rectum with extraperitoneal rectal anastomosis [17]. The most common indication for an LAR is rectal cancer. As with any oncologic procedure, proper patient selection is important. The primary goal is complete extirpation of the tumor and any involved lymphatics. Reestablishment of bowel continuity is a secondary goal. Preoperative staging with an endorectal ultrasound (ERUS) or magnetic resonance imaging (MRI) as well as distance from the anorectal ring dictate the ability to adequately resect using a transanal approach, LAR, or APR. Preoperative evaluation of patient comorbidity, fecal continence, and preoperative radiation are important influencing factors in the decision to reanastomose as well as the decision to create a diverting stoma proximal to the anastomosis.
Other indications for an LAR include inherited polyposis syndromes such as familial adenomatous polyposis (FAP) and proctitis refractory to medical management. Proctectomy for FAP is typically combined with total abdominal colectomy (TAC) for control of oncologic risk. LAR for medically refractory ulcerative colitis can be performed in combination with a TAC or may be part of a staged operation for ultimate control of disease.
Preoperative Workup
Although patients usually present to the surgeon with a confirmed endoscopic diagnosis of cancer, they may present with an initial complaint of rectal bleeding, pain, tenesmus, fullness, change in bowel habit, obstruction or obstipation, and/or weight loss. Many tumors are found incidentally either on screening colonoscopy or other imaging. Important features of the review of systems include weight loss, indicating a more urgent obstructive process; back pain or pain with defecation, indicating tumor eroding into the sacrum or sphincters; fecal or flatal incontinence; and baseline sexual and urinary function. The remaining portion of the history and physical should be focused on identifying other medical comorbidities, which may require further evaluation and risk stratification prior to surgical intervention. A thorough family history should be obtained to help identify those patients with a strong family history who may be at risk for a familial syndrome or inflammatory bowel disease.
The physical examination should include both a rectal exam and, if appropriate, a rigid proctoscopy. The rectal exam should specifically note size, mobility, fixation, and anatomic location of tumor including laterality as well as distance from the anorectal ring and anal verge. Rigid proctoscopy can be used to delineate the distance from the anal verge, length of tumor, and its relation to critical structures such as sphincters, peritoneal reflection, vagina, and prostate. If not done so prior, a full colonoscopy should be performed to identify the possibility of synchronous cancers, which occur 2–8 % of the time [18–21].
Preoperative staging is important in rectal cancer to assess the need for neoadjuvant therapy as well as determining the appropriate approach to surgical eradication of the tumor and the presence of regional lymph nodes the depth of invasion can be assessed by type out all ERU & MRI. A computed tomography (CT) of the chest, abdomen and pelvis should be obtained to evaluate the possibility of distant metastasis. Laboratory studies are obtained preoperatively based on other medical comorbidities. A carcinoembryonic antigen (CEA) should be checked before removal of the tumor to risk stratify the patient, since a higher CEA signifies a worse prognosis than those patients with the same stage and a normal CEA [22–25]. Also, an elevated preoperative CEA, which does not normalize postoperatively, warrants a workup for persistent disease.
Perioperative Preparation
Prior to the standard use of mechanical and preoperative antibiotics, the postoperative rate of infection was noted to be as high as 60 % [26]. Currently, a standard bowel preparation includes 1–3 days of clear liquids as well as some combination of hyperosmolar colonic irrigant such as polyethylene glycol or magnesium citrate and laxatives and/or enemas. Oral antibiotics are also used to decrease the bacteria count of the colon. Common choices are neomycin 1 g and erythromycin 1 g given at 5 p.m. and 10 p.m. the day before surgery. Metronidazole can be substituted to control a greater spectrum of anaerobes. Intravenous antibiotics are given per current Center for Medicare and Medicaid Services (CMS) recommendations [27]. According to the American Heart Association guidelines, the choice of perioperative intravenous antibiotic is broadened in those patients with cardiac prosthesis to include ampicillin [28]. The patient is also given a preoperative dose of 5,000 units of subcutaneous heparin. The abdomen is clipped as appropriate and cleaned using an antiseptic agent.
Surgical Technique
Operative Positioning and Exploration
The patient is brought to the operating room and general anesthesia is administered with the placement of an endotracheal tube. A foley catheter is sterilely placed within the bladder. The patient is then moved to the lithotomy position with the weight of the leg resting on the heel and appropriate support provided to alleviate pressure from both the low back and the lateral peroneal nerve of the lower leg. The arms are placed on arm boards bilaterally so as not to cause undue stretch on the brachial plexus (Fig. 20.3). Preoperative placement of urinary stents can aid in identification of bilateral ureters during dissection. A rectal exam is performed to reassess the tumor, its location, and possible fixation. The abdomen is prepped and draped in a sterile fashion, allowing appropriate access to the anus. A midline vertical subumbilical incision is made. If cephalad extension is required for mobilization of the splenic flexure, the incision should be continued on the contralateral side of any potential stoma. Once access to the abdomen is gained, the abdomen is explored for signs of metastatic disease including visualization and palpation of the peritoneal surfaces and the liver. The tumor is palpated within the pelvis to assess fixation and likelihood of invasion to surrounding structures. The abdominal self-retractor is used for adequate exposure. The patient is placed in slight Trendelenburg position and the small bowel is packed into the upper abdomen.
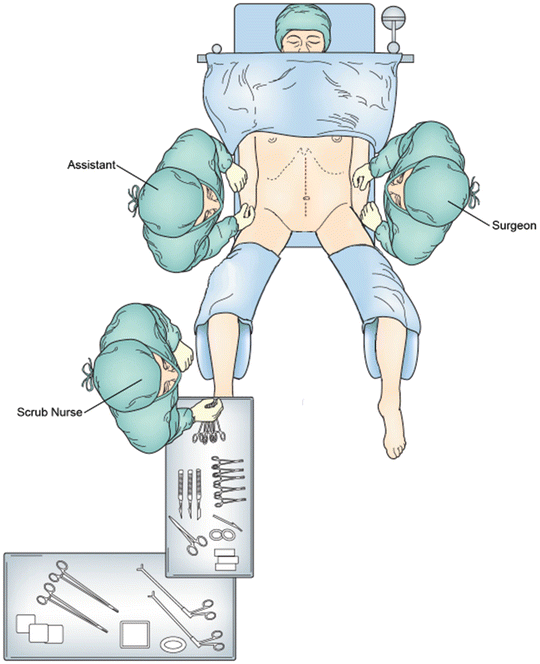

Fig. 20.3
Operating room setup with the patient in lithotomy position with the upper extremities placed on arm boards
Mobilization and Resection
Mobilization of the colon is begun laterally along the white line of Toldt (Fig. 20.4). The ureter is identified at the bifurcation of the common iliac artery. After mobilization of the colon is begun, the length of colon to be resected can be grossly estimated and, subsequently, the need to mobilize the splenic flexure assessed. During mobilization of the splenic flexure, tension on the left and transverse colon should be firm but not excessive and blunt dissection should be avoided to avoid inadvertent splenic injury. The omentum is freed from the transverse colon via sharp dissection in the avascular plane between the two structures.
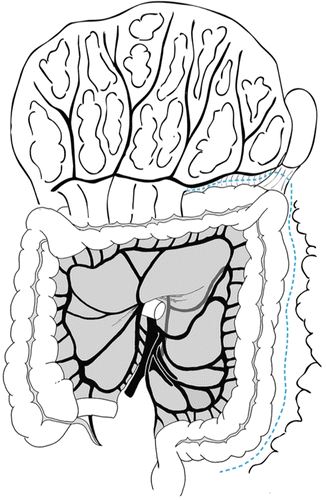

Fig. 20.4
Mobilization of the colon is begun laterally along the white line of Toldt
The sigmoid colon is manually elevated in the air and the mesentery is scored on both sides to the level of the sacral promontory. The inferior mesenteric artery (IMA) pedicle is isolated and the left colic is identified and spared, if appropriate. The superior hemorrhoidal artery is ligated at its takeoff from the IMA. Two large Kelly clamps are placed proximally and one is placed distally. The pedicle is then transected and doubly ligated. The bowel is subsequently transected at a level ensuring adequate blood supply to the remaining colon (Fig. 20.5). The method of transection depends on the type of anastomosis planned, i.e., stapled or hand-sewn. A TME is undertaken and crucial for adequate oncologic resection. After identifying the sympathetic nerves traveling over the pelvic brim, electrocautery is used to dissect in the posterior avascular, alveolar plane between the fascia propria of the rectum and the parietal fascia of the pelvic floor structures while the rectum is being retracted anteriorly. This, when undertaken accurately, allows for sparing of the autonomic nerves as well as the surrounding pelvic structures and results in a smooth, bilobed mesorectal specimen.
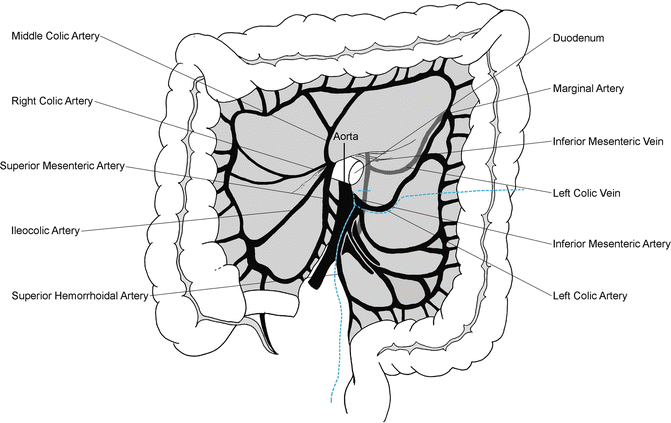

Fig. 20.5
Transection of mesentery vessels and colon. The superior hemorrhoidal artery is divided and the left colon and mesentery are divided at the junction of the descending and sigmoid colon
The posterior dissection is carried inferiorly through Waldeyer’s fascia to the level of the coccyx. A St. Mark’s abdominal retractor facilitates retraction of the rectum in the deep pelvis. The lateral and anterior dissection is undertaken using electrocautery. The anterior peritoneum is incised at the groove between the rectum and the anterior structures, either the cervix/vagina in women or the seminal vesicles in men. As stated, the lateral stalks contain the middle rectal vessels in 22 % of patients [8]. As these are divided, care should be taken to preserve the hypogastric plexus that lies on the pelvic sidewall, lateral to the seminal vesicles in men and the cardinal ligaments in women. In Japan, dissection of these lateral lymph nodes are purposefully pursued and has been routinely practiced since 1940 [29]. However, there is minimal, if any, oncologic benefit to lymphadenectomy in these lateral spaces [30–37]. Avoiding this dissection helps prevent significant postoperative problems with sexual potency and urination [34, 38–41]. During the anterior dissection, the planes are less distinct and the fat on the anterior mesorectum is thin. The anterior pelvic structures are elevated off the anterior rectal wall using a lipped St. Mark’s retractor. This dissection is carried through Denonvillier’s fascia. This fascia is taken off the anterior structures and kept with the specimen in an oncologic operation.
The distal point of bowel transection depends on the level of the tumor. Middle and distal rectal tumors require removal of the entire mesorectum. An upper rectal tumor requires transection of the rectum and mesorectum 5–6 cm below the level of the tumor. Tumor spread within the mesorectum rarely extends beyond 3–4 cm distally [42, 43]. In addition, multiple studies have shown that a 2 cm margin of the mucosa is likely more than adequate [11–16]. A rigid sigmoidoscopy may be helpful in identifying the tumor when it is not palpable, particularly after neoadjuvant therapy. Mobilization of the rectum can increase the distance of the tumor from the dentate line allowing an adequate distal margin with preservation of the sphincters, which may not have been perceived before mobilization of the rectum. Once the level of transection has been established, the mesorectum should be divided with bipolar thermal energy or direct suture ligation. The rectal wall can then be transected using a stapling device, either a thoraco-abdominal (TA) linear stapler or a curved contour stapler dependent on the width of the pelvis. The length of the proximal colon is then evaluated for construction of a tension-free anastomosis. If more length is needed, a variety of maneuvers can be employed. The splenic flexure can be further mobilized, the inferior mesenteric vein can be identified and transected, and the mesentery can be further ligated (at the risk of necessitating further colon resection).
Anastomosis
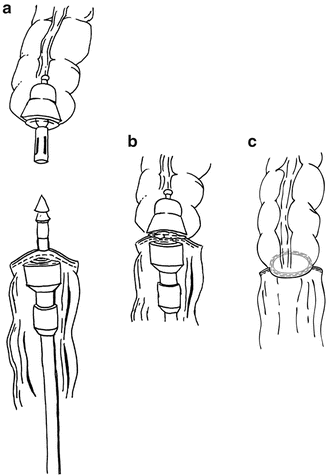
The size of end-to-end anastomosis (EEA) stapler is decided by the caliber of the colon. Dependent on the type of reconstruction planned (i.e., end-to-end or side-to-end), the anvil is either placed within the end of the colon after the staple line is amputated or a colotomy is made on the antimesenteric surface approximately 3 cm from the staple line and the anvil is placed within the colotomy. The anvil is secured with a purse-string using 3-0 prolene suture. The serosa of the colon is then cleaned of fat and small vessels within 1 cm of the shaft of the anvil to facilitate the bowel-to-bowel contact within the anastomosis.
Once the anvil is secured, the pelvis is then irrigated and excellent hemostasis is achieved prior to formation of the anastomosis, which will limit access to the deep pelvis. One member of the surgical team then gently dilates and places the lubricated circular stapler in the anus. The surgeon then guides the assistant to follow the curve of the sacrum and advance the stapler to the rectal staple line. Once in adequate position, the spike is deployed slowly ensuring proper positioning. We prefer to deploy the spike 2–3 mm posterior to the staple line to discourage the capture of any anterior structures in the EEA anastomosis. Once the spike is completely deployed, the anvil is brought to the pelvis and secured onto the spike making sure the mesentery and colon are properly oriented (Fig. 20.6). The stapling device is slowly closed, ensuring all extraneous tissue is removed from the anastomosis. The stapler is then fired, opened partially, and gently removed from the anus. The anastomotic “donuts” are examined to ensure identification of complete rings of tissue. If incomplete rings are found, the anastomosis should be scrutinized for any compromise. An air test of the anastomosis is then performed. The surgeon fills the pelvis with irrigant and clamps the colon proximal to the anastomosis while the assistant insufflates the rectum with air via rigid proctoscopy. If bubbling is identified, the anastomosis is reinforced or refashioned.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








