Brian Rayner, Karen E. Charlton, Estelle V. Lambert
Nonpharmacologic Prevention and Treatment of Hypertension
Lifestyle changes, including a combination of increased fat and refined carbohydrate intake and reduced physical activity, have resulted in an epidemic of obesity, type 2 diabetes mellitus, and hypertension. The epidemic is evident worldwide and often is greatest in underserved and indigenous populations. Adoption of healthy lifestyles is critical for both the prevention of high blood pressure (BP) and its management. According to the Seventh Report of the Joint National Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JNC 7 Report),1 lifestyle interventions lower BP, enhance efficacy of antihypertensive medication, and lower overall cardiovascular (CV) risk. The lifestyle changes that are widely agreed to lower BP and CV risk are (1) smoking cessation, (2) weight reduction, (3) moderation of alcohol intake, (4) physical exercise, (5) reduction of salt intake, (6) increase in fruit and vegetable intake, and (7) decrease in saturated and total fat intake.1 Interventions may have efficacy similar to that of single-drug therapy (Table 35-1). However, lifestyle changes should not delay the initiation of drug therapy in patients at higher CV risk.
Table 35-1
Lifestyle modifications for prevention and management of hypertension (JNC 7).
BMI, Body mass index; BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension.
| Lifestyle Modifications for Prevention and Management of Hypertension | ||
| Modification | Recommendation | Average Systolic BP Reduction Range Achieved with Intervention* |
| Weight reduction | Maintain normal body weight (BMI = 18.5-24.9 kg/m2) | 5-20 mm Hg/10 kg |
| DASH eating plan | Adopt a diet rich in fruits, vegetables, and low-fat dairy products with reduced content of saturated and total fat | 8-14 mm Hg |
| Dietary sodium restriction | Reduce dietary sodium intake to 100 mmol/day (2.4 g sodium or 6 g sodium chloride) | 2-8 mm Hg |
| Aerobic physical activity | Regular aerobic physical activity (e.g., brisk walking) at least 30 min/day, most days of the week | 4-9 mm Hg |
| Moderation of alcohol consumption | Men: limit to 2 drinks† per day; women and lighter-weight persons: limit to 1 drink per day | 2-4 mm Hg |
* Effects are dose and time dependent.
† One drink = 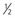 oz or 15 ml ethanol (e.g., 12 oz (360 ml) beer, 5 oz (150 ml) wine, 1.5 oz (45 ml) 80-proof whiskey).
oz or 15 ml ethanol (e.g., 12 oz (360 ml) beer, 5 oz (150 ml) wine, 1.5 oz (45 ml) 80-proof whiskey).
(From reference 1.)
Prevention
The importance of primary prevention has been underscored by the recognition that (1) the treatment of hypertension is expensive, (2) the control of BP in hypertensive individuals does not restore CV risk to normal, and (3) the majority of hypertensive individuals do not reach goal BP readings. The most important individuals to target are those with prehypertension (defined by JNC 7 as 120 to 139/80 to 89 mm Hg; see Chapter 33).1 Individuals with prehypertension have increased prevalence of early vascular damage, increased risk of incident hypertension, and increased risk of CV events compared with those who have optimal BP levels (<120/80 mm Hg).2 The JNC 7 Report recommends lifestyle changes in all patients with prehypertension to prevent hypertension and to reduce CV risk.1
The contribution of body weight, physical inactivity, and dietary factors to the prevalence of hypertension in Europe and the United States has been quantified in a meta–regression analysis.3 Being overweight made the largest contribution to hypertension, with population-attributable risk percentage (PAR%) between 11% (Italy) and 25% (United States). PAR% was 5% to 13% for physical inactivity, 9% to 17% for high sodium intake, 4% to 17% for low potassium intake, and 4% to 8% for low magnesium intake. The impact of alcohol was small (2% to 3%) in all populations. PAR% varied among populations for inadequate intake of calcium (2% to 8%) and inadequate intake of fish fatty acids (3% to 16%).
Weight Loss
Obesity is epidemic throughout the world, and 65% of the adult population in the United States is either overweight, with body mass index (BMI) of 25.0 to 29.9, or obese, with BMI of 30 or higher.4 Obese individuals have a threefold increased prevalence of hypertension. Possible mechanisms for obesity-induced hypertension include overactivity of the sympathetic nervous system (SNS), hyperinsulinemia (which may increase renal sodium reabsorption), increased leptin, hyperuricemia, activation of the renin-angiotensin system (RAS), and sleep apnea. Abdominal or visceral obesity is a greater predictor of both hypertension and CV risk than is a predominant lower body fat distribution. Abdominal obesity is defined as a waist circumference greater than 88 cm (>35 inches) in women and greater than 102 cm (>40 inches) in men. These reference values were developed in Caucasian populations, and differing criteria may be more appropriate for other ethnic groups.
In obese hypertensive patients or those with high-normal BP, weight loss of as little as 4 to 5 kg (~9 to 11 lb) is often associated with a significant reduction in BP. Weight loss is one of the most effective nonpharmacologic interventions to reduce BP.5 A meta-analysis has demonstrated that a weight reduction of 5.1 kg reduced systolic BP by 4.4 mm Hg and diastolic BP by 3.6 mm Hg.6 A rule of thumb is that for every kilogram lost, there is a reduction of 1 mm Hg in both systolic and diastolic BP. To minimize the risk of relapse and to maintain sustainability of the weight loss program, the initial target should be 5% to 10% of current weight, or 1 to 2 BMI units. Marked oscillations in weight should be avoided because this increases the risk for development of hypertension in obese, normotensive persons.7 A randomized trial of the effectiveness of four popular diets on sustained weight loss and CV disease risk reduction concluded that a variety of diets can similarly reduce weight and BP, but only a minority of individuals can sustain high dietary adherence. High-protein, low-carbohydrate diets, advocated for weight loss by the media and lay public, are associated with an overall increase in CV events in women.8
Weight reduction should be accompanied by recommendations to increase physical activity unless it is contraindicated. Bariatric surgery and pharmacologic interventions for weight loss (e.g., orlistat) may be useful to achieve weight loss in some patients, but always as an adjunct rather than a substitute for lifestyle modification.
Physical Activity
Physical inactivity is associated with at least a 1.5- to 2-fold higher risk of hypertension and coronary heart disease.9 Regular physical activity lowers all-cause morbidity and mortality and provides the basis for public health recommendations to exercise at least 30 minutes per day. A recent review reported that patients with high BP who participated in any level of physical activity had a reduced risk (by 16% to 67%) of CV mortality, whereas a greater than twofold increase in CV mortality risk was observed in nonactive individuals.10 Regular physical activity in childhood was inversely correlated with diastolic BP, even in children as young as 5 years.11
Exercise Training Dose Response
In a meta-analysis of studies involving more than 1500 patients, exercise training in normotensive individuals has been shown to reduce systolic and diastolic BP by 3.0 ± 1 and 1.7 ± 1 mm Hg, respectively.12 The rate of progression from prehypertension to hypertension in middle-aged and older male veterans was prevented when exercise capacity exceeded 8.5 peak metabolic equivalents (METs).13 In hypertensive patients, the effect of exercise training is even more marked: a reduction of 7.8 ± 3.5 and 5.8 ± 2.0 mm Hg for systolic and diastolic BP, respectively. Studies in hypertensive patients have shown that the benefit of exercise on BP is maximal with 90 minutes of exercise per week, after which there was no further improvement.14 Furthermore, only a modest amount of exercise was needed to reduce BP in patients with hypertension (>30 min/wk). Regular exercise prevents the development of left ventricular hypertrophy (LVH) that is independent of BP in young, stage 1 hypertensive patients.15
Fagard12 found no benefit of increasing exercise intensity on BP reduction with exercise training, as long as intensity ranged between 40% and 70% of maximal, age-predicted heart rate. Exercise of higher intensity (75% maximum) was associated with a more marked and prolonged reduction in BP in the postexercise window compared with lower-intensity exercise (50% maximum).16
Mechanisms
In the acute postexercise period, the reduction in BP has been linked to a sympathetic inhibition and increased release of vasodilator substances.17 The mechanisms by which chronic exercise lowers BP are less well understood but include reductions in systemic vascular resistance (SVR) secondary to neurohumoral and structural adaptations. Chronic exercise is also associated with a loss of weight and a reduction of serum uric acid levels, both of which could reduce BP.
Antihypertensive Medication and Guidelines for Exercise
Box 35-1 provides exercise guidelines for patients with hypertension.18 β-Blockers decrease exercise tolerance. β-Blockers and diuretics may also alter thermoregulation in hot environments and provoke hypoglycemia. Patients using these medications should be educated about exercising in the heat, clothing, adequate hydration, and methods to prevent hypoglycemia.19,20 Angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers may be better suited for patients who exercise frequently or athletes with hypertension.
For patients undergoing supervised exercise training, the monitoring of postexercise BP may be helpful so that medications may be adjusted appropriately, considering the likelihood of postexercise hypotension. Particular care should be taken with patients planning to exercise in a hot environment.
Diet
Salt Intake
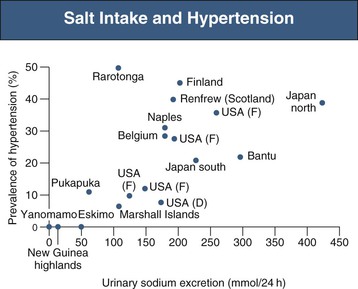
Epidemiologic studies have demonstrated that the prevalence of hypertension is directly related to the dietary salt intake in societies throughout the world in whom salt intake is above 50 to 100 mmol/day, or 3 to 6 g sodium chloride (NaCl)21 (Fig. 35-1). Where daily intake is below that range, hypertension is rare. Salt intake also plays an important role in age-related increase in BP22 (Fig. 35-2). Not all individuals respond similarly to high salt intake. “Salt sensitivity” describes a group of individuals who significantly decrease or increase their BP during periods of salt restriction or loading, respectively. Despite the definition remaining in the research rather than the clinical domain, the following groups of patients tend have increased susceptibility to salt sensitivity (salt-sensitive hypertension):
• Obese persons (not in all studies)
• Patients with type 1 or type 2 diabetes
• Patients treated with calcineurin inhibitors
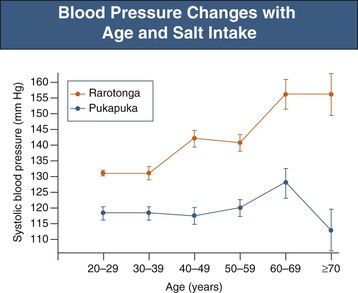
Salt sensitivity is observed in 75% of hypertensive African Americans compared with about 50% of hypertensive Caucasians23 and increases with age in both normotensive and hypertensive populations24 (Fig. 35-3). Response to salt restriction is predicted by polymorphisms in the G protein–coupled receptor kinase 4 (GRK-4) gene, which regulates Na+ excretion in the proximal convoluted tubule.25 Putative mechanisms for salt sensitivity are alterations in circulating levels of (or renal responses to) atrial natriuretic factor, kallikrein, prostaglandins, and nitric oxide (NO); increased levels of norepinephrine; abnormal suppression of both renin and aldosterone; genetic mechanisms; congenital reduction in nephron number; and acquired renal microvascular and tubular injury.23
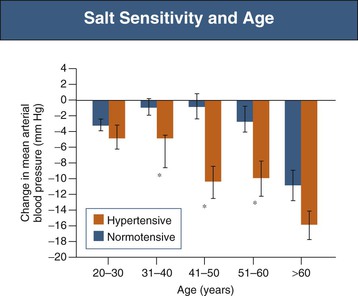
The effects of salt restriction on BP have been demonstrated in several well-designed intervention studies.26 In a randomized controlled trial (RCT) in British people age 60 to 78 who were not receiving antihypertensive medication, modest salt restriction from 10 g to 5 g per day resulted in a reduction in BP of 7.2/3.2 mm Hg during a 4-week period in both normotensive and hypertensive subjects.27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


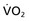 peak).
peak).





