Chapter 90 NEUROENDOCRINE ROLE IN INTERSTITIAL CYSTITIS AND CHRONIC PELVIC PAIN IN WOMEN
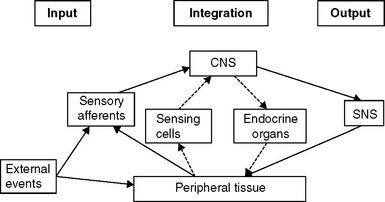
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” The awareness of acute pain motivates those experiencing it toward withdrawal and guarding to permit avoidance of further injury and activation of repair processes. In some cases of chronic pain, however, the situation may be somewhat different, in that the severity of signs loses its usual tight correlation with symptoms. In chronic visceral pain states such as interstitial cystitis (IC) and chronic pelvic pain (CPP), the endocrine system also may be involved in maintaining the sensation of pain (Fig. 90-1),1,2 although the extent to which (or in which patients) this results from an underlying genetic or developmental predisposition and to what extent it results from the etiology of the disease process cannot yet be determined.
IC is a lower urinary tract syndrome of unknown cause and no generally accepted treatment.3 The symptoms of IC include variable combinations of pain referable to the urinary bladder, as well as increased frequency and urgency of urination. IC may affect more than 700,000 American women,4 and a significant (potentially comparable) number of men diagnosed with sterile prostatitis or prostatodynia.5 The quality of life of IC patients is significantly degraded; in one study, these patients scored much lower (P < .001) than healthy control subjects did in all eight domains of health assessed by the Medical Outcomes Study Short Form-36 Health Survey.3
Since 1993, my laboratory has investigated a common lower urinary tract disorder of domestic cats, feline interstitial cystitis (FIC), that represents a naturally occurring model of IC. We have found that cats with FIC meet all the criteria promulgated by the National Institutes of Health for diagnosis of IC that can be applied to animals, and we have shown that cats with FIC and humans with IC have comparable bladder, sensory afferent, central, sympathetic, and endocrine abnormalities, to the extent they have been investigated. Recent findings in these cats3,6 are consistent with and extend the neuroendocrine abnormalities identified in humans with IC.
The National Institutes of Health classifies IC as a CPP syndrome, which has been defined as nonmenstrual pain of at least 6 months’ duration that is severe enough to cause functional disability or necessitate medical or surgical treatment.7 As many as 39% of women of reproductive age seen by primary care physicians report the presence of CPP “always,” “often,” or “sometimes.”8 Women with CPP use more medications, have more nongynecologic operations, and are five times more likely to have a hysterectomy than are women without CPP. These patients also are more likely to have a history of abuse and to suffer from depression, impaired sexual functioning, and reduced overall quality of life.9
A recent comprehensive review7 listed some 70 disorders (15 extrauterine and 8 uterine, 11 urologic, 8 gastrointestinal, 17 musculoskeletal, and 11 “other”) that may be associated with CPP in women, including IC, irritable bowel syndrome, and fibromyalgia. The comorbidity of some of these diseases was suggested by the results of a recent mail questionnaire survey in England, which found that 24% of women aged 18 to 49 years reported CPP during the previous 3 months. Of these women, 52% had CPP only, 24% had CPP and irritable bowel syndrome, 9% had CPP and urinary frequency and urgency, and 15% had all three.10 These results suggest that patients with CPP have variable combinations of organ involvement, raising the question of the extent to which a different etiology affects each organ individually, or whether some common underlying etiology affects a variety of organs, which then respond in their own characteristic ways.
Two subtypes of IC currently are recognized based on cystoscopic evaluation of the bladder. In most patients (90%), only submucosal petechial hemorrhages (glomerulations) are observed (type I), whereas mucosal (“Hunner’s”) ulcers, with or without glomerulations, are identified in a minority (type II). The two types also appear to differ in patient epidemiology, histologic findings, and response to treatment, further suggesting that they may be distinct entities.11 One important difference between the two types is that many patients with type II IC report significant symptomatic relief after supratrigonal cystectomy and cystoplasty, whereas the pain in patients with type I IC is not usually diminished by this procedure.12 This difference in patient response to removal of the bladder may provide an important clue to the underlying causes of pain associated with IC: the cause of the pain in patients with type II disease may be nociceptive, whereas the pain of patients with type I IC may be neuropathic.
Nociceptive pain results from persistent activity of sensory afferent fibers innervating the affected area and is relieved by removal of the stimulus. Examples of nociceptive pain include toothache, which is relieved by extraction, and osteoarthritis of the hip joint, which is relieved by hip replacement.13 In contrast, neuropathic pain arises from some abnormality related to the nervous system; although generally attributed to a body structure, it can remain after desensitization of nociceptive afferents14,15 or even removal of the structure.16,17 Such results have been reported for endometriosis, where removal of identified abnormalities does not always lead to resolution of the CPP.18
THE STRESS RESPONSE SYSTEM
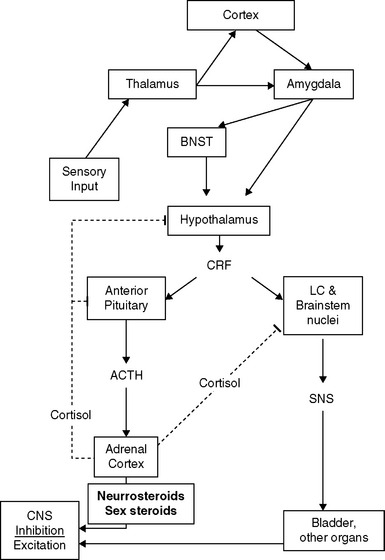
The presence of neuropathic pain may be related to abnormalities and imbalances of the neuroendocrine system, which is activated in response to threats to homeostasis. One commonality among some IC and CPP patients appears to be a relative predominance of activation of the sympathetic nervous system (SNS) limb of the stress response system (SRS), compared to the responses of the hypothalamic-pituitary-adrenal (HPA) and -gonadal (HPG) axes.19 A schematic diagram of some of the features this complex system20 is presented in Figure 90-2. Once the system is stimulated by central nervous system structures responding to sensory inputs (conscious or unconscious21) that are perceived as a threat to homeostasis, corticotropin-releasing factor (CRF) is released from the paraventricular nucleus of the hypothalamus. CRF acts as a neurotransmitter, to activate sympathetic premotor neurons in the pontine locus coeruleus and brainstem nuclei, and as a hormone, to stimulate the anterior pituitary. In some patients, the SNS arm of the response appears to be uncoupled from the HPA and HPG axes in that SNS outflow increases in the absence of activation of the HPA axis and in the presence of reduced HPG function.
Sympathoneural Output
Even though the neuroendocrine features of the stress response have not been thoroughly studied in humans with IC and CPP, the available data support the presence of a comparable abnormality in at least a subset of these patients. Although plasma catecholamine concentrations have yet to be reported patients with IC, the findings of abnormal vasomotor tone,22 increased density of bladder neurons staining for tyrosine hydroxylase (the rate-limiting enzyme for catecholamine synthesis),23,24 and increased urine norepinephrine excretion25 that have been reported suggest increased SNS activity.
Recent studies have begun to map the pathways that transduce activation of the SRS into cellular dysfunction via the SNS. Events external to the central nervous system, from both within and without the body, are transmitted to the brain by the sensory neurons. These signals are conveyed to the thalamus, where they are evaluated and usually forwarded to the cerebral cortex for further processing before activation of the appropriate motor program (see Fig. 90-2).26 Potentially threatening events, however, can activate the SRS directly via the thalamic activation of the amygdala, bypassing cortical inhibitory control.27
Activation of the SNS results in sympathoneural release of norepinephrine. A 2003 study28 traced this pathway from the external environment through to norepinephrine-mediated induction of the transcription factor nuclear factor kappa B (NF-κB), which is thought to play a role in mediating the urothelial inflammatory response of IC.29 In vitro stimulation of human promonocytic (THP-1) cells with physiologic amounts of norepinephrine for 10 minutes resulted in a dose- and time-dependent induction of NF-κB and NF-κB-dependent gene expression; only norepinephrine induced this response, which was reduced by both α1– and β-adrenergic receptor antagonists. The authors concluded that norepinephrine-mediated activation of NF-κB represented a downstream effector of the neuroendocrine response to stressful psychosocial events, linking changes in the environment to a bewildering array of cellular responses through activation of the SRS.30
Activation of the SRS also can increase epithelial permeability, permitting environmental agents greater access to sensory afferent neurons,31 which could result in both increased sensory afferent firing and local inflammation. Sympathetic neural-epithelial interactions appear to play an important role in urothelial permeability. For example, Birder and colleagues showed that application of β-adrenergic receptor agonists to urinary bladder strips induced release of nitric oxide (NO) from urinary bladder epithelium, raising the possibility that norepinephrine from adrenergic nerves in the bladder (which may not be present in normal individuals)23,24 or circulating catecholamines could influence bladder function by acting on β-adrenergic receptors in the urothelium to release NO and possibly other neurotransmitters, such as adenosine triphosphate (ATP).32 Application of capsaicin, the pungent principle in hot peppers, also resulted in release of NO from epithelium as well as nervous tissue in the urinary bladder.33 In light of reports that NO may increase urothelial permeability,34,35 these results suggest that some of the sympathetically mediated alterations in permeability may be mediated by norepinephrine via this mechanism.
The increased permeability related to increased SNS activation does not require direct interaction with epithelial cells, nor is it restricted to the urinary bladder.36 Moreover, neural release of norepinephrine37 is but one of a variety of mechanisms whereby SRS-induced increases in efferent SNS output can activate local inflammatory cells such as mast cells, which can in turn also increase epithelial permeability.36 Afferent sensory neurons, too, may increase epithelial permeability by releasing neurotransmitters at the peripheral process of the nerve via sympathetic-sensory coupling, dorsal root reflexes, and axon reflexes.38 Recently, increased sensitivity to potassium chloride instillation, a test thought by some to indicate increased urothelial permeability, was reported in 244 female patients with CPP. Eighty-one percent of patients, with clinical diagnoses that included endometriosis, vulvodynia, and pelvic pain, showed a positive (painful) response to potassium instillation into the bladder.39 However, patient’s sensitivity to potassium instilled into other organs (vagina, uterus, colon, or peritoneum) was not assessed, so the specificity of the response cannot be evaluated. This may be an important control procedure, because a number of studies have reported that abnormalities in one visceral organ may affect responses in another, a process called viscerovisceral convergence.40–42 In cats with FIC, we recently reported43,44 sensitization and abnormal properties of dorsal root ganglion cells of axons that provide innervation not only to the bladder but throughout the lumbosacral (L4-S3) region, suggesting that generalized hypersensitivity may a mediating mechanism for viscerovisceral convergence in animals with naturally occurring as well as induced disease.
Moreover, one must recall that the presence of inflammation, or altered permeability, is not well correlated with pain, as anyone who has had a superficial bruise knows from personal experience. In the bladder, we have reported the presence of submucosal petechial hemorrhages in cats with no signs referable to the lower urinary tract,45 and others have identified petechial hemorrhages in healthy women,46 as well as urothelial disruption and increased presence of inducible nitric oxide synthase (iNOS), and presumably increased permeability, in elderly men with bladder outlet obstruction.47
Moreover, emotional and environmental factors such as stress or depression can modulate the experience of pain through descending pathways from the midbrain.48 Therefore, even the recently reported increased firing rate of afferent nerves noted in cats with FIC49 could result in differences in perceived sensations arising from the bladder, depending on the effects of the emotional state of the animal on descending inhibitory and facilitory balance.
Hypothalamic-Pituitary-Adrenal Axis
The HPA axis of the SRS acts at multiple levels to coordinate and modulate the body’s response to perceived threats. Glucocorticoids tend to antagonize the effects of the SNS, both centrally and peripherally,20 and they appear to play a complex role in epithelial permeability. Corticosterone has long been known to decrease capillary permeability to proteins in the brain,50 skin,51 and lung,52 and cortisol has been shown to reduce in vitro permeability by enhancing tight junction integrity.53 The effects of stress on glucocorticoid-mediated alterations in permeability are more complex and may be dose dependent. For example, stress created by a brief forced swim increased the permeability of the blood-brain barrier in adult FVB/N mice, whereas no evidence of a stress-potentiated effect was found when restraint, forced swim, or a combination of restraint and forced swim stressors were applied to Long-Evans or Wistar rats.54 Moreover, stress-induced increases in intestinal epithelial permeability disappeared after adrenalectomy or pharmacologic blockade of glucocorticoid receptors, and dexamethasone treatment of control animals increased gastrointestinal permeability and mimicked the effects of stress.55 Differences in tissues studied, rodent strain, type of stress, glucocorticoid studied, and the specifics of the experimental protocol all could influence interpretation of these discordant results.
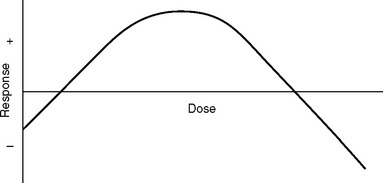
The response to glucocorticoids also is likely to be hormetic (Fig. 90-3); that is, there is an inverse-U–shaped function wherein both deficiencies and excesses may produce abnormalities, which sometimes are relatively similar, further complicating interpretation of the results.56 Glucocorticoids also tend to inhibit activation of NF-κB.57,58 This and other adrenocortical steroid-related protective mechanisms, such as inhibition of the SRS and modulation of neuronal excitability,59–61 may be less efficient in states of reduced function of cortisol and other steroids (see later discussion) such as IC and other stress-related bodily disorders.62
In a 2002 study of IC patients and healthy controls, Lutgendorf and colleagues63 reported that, although mean urinary or salivary cortisol did not differ between the groups, IC patients who had higher morning salivary cortisol concentrations had significantly reduced pain and urgency, and those with higher urinary free cortisol concentrations reported less overall symptomatology (P < .05). This relationship also was observed when comorbid conditions such as fibromyalgia, chronic fatigue syndrome (CFS), and rheumatoid arthritis were controlled for. Patients with morning salivary cortisol concentrations less than 12.5 nmol/L (0.45 μg/dL) were 12.8 times more likely to report high urinary urgency than those with values above this cutoff point. An increased ratio of adrenocorticotropic hormone (ACTH) to cortisol also was reported in women with IC by Lutgendorf and associates.64
Hypocortisolism has been reported in women with CPP, CFS and a variety of other disorders, often related to increased activity of the SRS.62 The causes of the decrease in cortisol have yet to be identified in patients with IC but have been investigated in patients with CPP62,65 and CFS.66
Although diagnostic laparoscopy may be normal in some patients with CPP, psychological studies have identified a high frequency of psychopathology and increased prevalence of chronic stress and traumatic life events, such as sexual and physical abuse, in women with CPP, suggesting a relationship between post-traumatic stress disorder (PTSD) and CPP. Heim and colleagues65 explored stress history, psychopathology, and HPA axis alterations in 16 female patients with CPP and 14 pain-free, infertile controls. An increased prevalence of abuse experiences and PTSD was identified in women with CPP, as well as a higher total number of major life events, although symptoms of depression were within the normal range. Women with CPP also had normal to low diurnal salivary cortisol concentrations and normal plasma ACTH but reduced salivary cortisol response to a CRF stimulation test and enhanced suppression of salivary cortisol by dexamethasone. The authors concluded that a lack of protective properties of cortisol may be of relevance for the development of bodily disorders in chronically stressed or traumatized individuals, although other hormones were not measured. It is not necessary to show such extreme examples of abuse to find correlations between early adverse experience and disease, which also may result from environmental instability and parenting variables.67,68
In patients with CFS, Demitrack and associates66 concluded, after comprehensive study of the HPA axis, that the data were most compatible with a mild central adrenal insufficiency secondary to either a deficiency of CRF (although this was not identified) or some other central stimulus to the pituitary-adrenal axis. Scott and coauthors later reported that the adrenal glands of patients with CFS were some 50% smaller than those of control subjects based on computed tomography.69 Although they studied patients who had low cortisol responses to ACTH, these authors subsequently found comparable results in CFS patients with normal cortisol responses to ACTH.70 A more recent study did not find any difference from normal in adrenal gland volume in another group of CFS patients,71 leaving the question of the role of adrenal volume in the observed abnormalities still open. Additionally, Kizildere and associates72 recently identified a β-adrenergic receptor–mediated inhibition of CRF-stimulated adrenal steroid secretion in healthy humans. They found that administration of 10 mg propranolol (a nonspecific β-adrenergic receptor antagonist) 2 hours before administration of 100 μg human CRF decreased heart rate and diastolic blood pressure by 20%. Propranolol treatment also reduced plasma ACTH concentrations by about 40% and increased serum cortisol by approximately 70%, which decreased the ACTH-to-cortisol ratio by twofold. These results suggest that increased sympathetic tone also may reduce adrenocortical responsiveness to ACTH. Moreover, ACTH release can be enhanced by α-adrenergic receptor activation, as well as by vasopressin, which also can modulate SRS activity.73
There is convincing evidence that the adrenal cortex is hypoactive in some circumstances in a variety of chronic disorders other than IC and CPP, including asthma, chronic fatigue syndrome, fibromyalgia, panic disorder, PTSD, and rheumatoid arthritis. Moreover, flares in disease activity in these disorders have been related to stress. Although hypocortisolism appears to be a frequent and widespread phenomenon, the nature of the underlying mechanisms and the homology of these mechanisms within and across clinical groups remain speculative. Potential mechanisms underlying the observed hypocortisolism include dysregulation of function at any level of the HPA axis, genetic vulnerability, previous stressful experiences, and individual coping and personality styles.62
In neuroendocrine investigations comparing healthy cats to cats with FIC in a basal state, we found higher plasma catecholamine concentrations in cats with FIC but could not identify a difference in response of ACTH and cortisol to CRF between affected and healthy cats.74 Based on some anomalous responses to a naturalistic stressor obtained during experiments with a CRF receptor antagonist in cats with FIC,75 we began to look more closely at adrenal function during activation of the SRS and found increased concentrations of CRF76,77 and ACTH75 in the absence of a comparable increase in plasma cortisol concentrations, suggesting the presence of mild primary adrenal insufficiency or decreased adrenocortical reserve. We also found that adrenal gland size was significantly smaller in cats with FIC than in healthy cats.78 Microscopic examination of the glands did not reveal any obvious hemorrhage, inflammation, infection, fibrosis, or necrosis as causes of the reduced size. The primary abnormality identified was a reduced size of the fasciculata and reticularis zones of the adrenal cortex. These results suggest that any adrenocortical abnormality might be unmasked more readily in response to a moderate, salient stressor and may not be identifiable in patients studied under basal circumstances, a conclusion about these systems that has also been drawn by others studying human patients.79,80
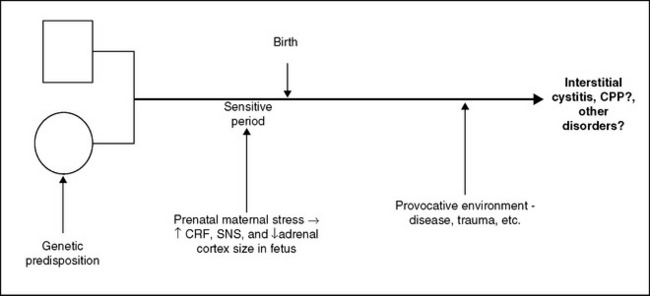
The simplest explanation for the combination of increased CRF, ACTH, and SNS activity in the presence of reduced adrenocortical response and small adrenal fasciculata and reticularis zones without other apparent abnormalities seems to be the presence of an underlying genetic disorder or developmental anomaly (or some combination of the two). These relationships are depicted in Figure 90-4. When a woman is exposed to a sufficiently harsh stressor during pregnancy, the hormonal products of the ensuing stress response may cross the placenta and affect the course of fetal development. Prenatal and postnatal stressors can result in persistently increased central CRF activity in animals.81 For example, in both continuous and last-trimester paradigms, prenatal dexamethasone (0.1 mg/kg) treatment increased CRF messenger RNA levels specifically in both the hypothalamus and central nucleus of the amygdala, key loci for the effects of the neuropeptide on the expression of fear and anxiety.82
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree