David A. Bushinsky, Rebeca D. Monk
Nephrolithiasis and Nephrocalcinosis
Kidney stones are common and are associated with significant morbidity. Nephrolithiasis refers to stone formation within the renal tubules or collecting system, although calculi are often found within the ureters or in the bladder.1–3 Most renal calculi are calcium oxalate, calcium phosphate, struvite, uric acid, and cystine. Clinical presentation varies from asymptomatic small stones to large, obstructing staghorn calculi that impair renal function and cause chronic kidney disease (CKD). The severity of stone disease depends on the pathogenesis as well as stone type, size, and location. Nephrolithiasis classically presents as ureteral colic, but may also commonly present with hematuria or urinary tract infection (UTI). Diffuse renal parenchymal calcification is termed nephrocalcinosis. These calcifications are usually calcium phosphate or calcium oxalate and may deposit in the renal cortex or medulla, depending on the cause.
Nephrolithiasis
Epidemiology
Kidney stones are common in industrialized nations, with an annual incidence of over 1/1000 persons and a lifetime risk of forming stones of approximately 5% in women and approximately 13% in men.1,3,4 In the United States the prevalence of nephrolithiasis increased from 3.2% in the late 1970s to 5.2% in the 1990s5 and further to 8.8% in the 2000s, in parallel with the rising incidence of obesity, insulin resistance, and type 2 diabetes mellitus.6–8 Incidence peaks in the third and fourth decades and prevalence increases with age until approximately 70 years in men and 60 years in women.5 Factors that determine renal stone prevalence include age, sex, race, and geographic distribution. In the United States, Caucasians are more likely to develop renal stones than African Americans, Hispanics, or Asian Americans, but the prevalence is rising in non-Caucasians as well.8 Men are more prone to stone formation than women.8 In the United States the tendency for the development of stones also depends on geographic location, with an increasing prevalence from north to south and, to a lesser degree, from west to east. Increased environmental temperatures have been associated with increased stone formation rates.9 The increase in nephrolithiasis rates may be a result of the greater sunlight exposure leading to an increase in insensible losses through sweating, and more concentrated urine.4,10 The higher urine calcium excretion in a smaller urine volume will increase the risk for supersaturation for calcium-containing crystals, thereby promoting stone formation.
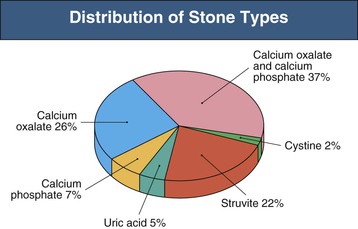
Stone type varies with worldwide geography and genetic predisposition. In the Mediterranean and Middle East, 75% of stones are composed of uric acid, whereas in the United States the majority of stones are calcium oxalate or calcium phosphate (>70%), with less than 10% being pure uric acid stones. Magnesium ammonium phosphate (struvite) stones account for 10% to 25% of stones formed (with a higher incidence in the United Kingdom), and cystine stones constitute 2% of all stones formed (Fig. 59-1).11
Outbreaks of kidney stones can also occur as a result of dietary supplements or medications. A large number of Chinese infants and toddlers developed kidney stones, and in some, renal failure, because of obstruction caused by stones. This outbreak was associated with melamine contamination in infant formulas and powdered milk as a means of raising the apparent concentration of protein in the products.12
Pathogenesis
Stones occur in urine that is supersaturated with respect to the ionic constituents of the specific type of stone. Supersaturation is dependent on the product of the free ion activities of stone components rather than on their molar concentrations. Whereas an increasing concentration of crystal components increases their free ion activity, other factors diminish it. When calcium and oxalate are dissolved in pure water, for example, the solution becomes saturated when the addition of any more calcium or oxalate does not result in further dissolution. However, urine, unlike pure water, contains numerous ions and molecules that can form soluble complexes with the ionic components of the stone. The interactions with these other solutes (e.g., citrate) may result in a decrease in free ion activity, which allows the stone constituents to increase in total concentration to levels that would normally cause stone formation in water. Urinary pH also influences free ion activity. The level of chemical free ion activity in which stones will neither grow nor dissolve is referred to as the equilibrium solubility product, or the upper limit of metastability. Above this level, the urine will be supersaturated and any stone present will grow in size.
When the solution becomes supersaturated with respect to a solid phase, ions can join together to form the more stable, solid phase, a process termed nucleation. Homogeneous nucleation refers to the joining of similar ions into crystals. The more common and thermodynamically favored heterogeneous nucleation results when crystals grow adjacent to crystals or other substances in the urine such as sloughed epithelial cells. Calcium oxalate crystals, for example, can nucleate with uric acid crystals. These small crystals may then aggregate to form larger stones, which would pass into the urine, causing crystalluria, if they did not anchor to the urothelium.
Calcium oxalate crystals anchor on areas of calcium phosphate deposits termed Randall plaques, which are located in the renal papillae and are composed of apatite (a complex of mostly calcium and phosphate) crystals. The apatite appears to originate around the thin loop of Henle, in the tubular basement membrane, and extends into the interstitium without filling the tubular lumen or damaging the tubular cells. Calcium oxalate crystals attach to Randall plaques, allowing significant stone growth.13–15 Clinically apparent stone disease occurs when the calcium oxalate crystals break off from the Randall plaques and cause injury or obstruction.
Clinical Manifestations
The two most characteristic symptoms of nephrolithiasis are pain and hematuria. Other presentations include UTIs and acute kidney injury (AKI) caused by obstructive uropathy if stones cause bilateral renal tract obstruction or unilateral obstruction in a single functioning kidney (Table 59-1).
Table 59-1
Clinical presentations of nephrolithiasis.
| Clinical Presentations of Nephrolithiasis | |
| Presentation | Characteristics |
| Pain | Ureteral colic, loin pain, dysuria |
| Hematuria | — |
| Urinary tract infection | Recurrent, chronic infection, pyelonephritis |
| Asymptomatic urine abnormality | Microhematuria, proteinuria, sterile pyuria |
| Interruption of urinary stream | — |
| Calculus anuria | — |
Pain
The classic presentation of pain in patients with nephrolithiasis is ureteral colic. Pain is of abrupt onset and intensifies over time into an excruciating, severe flank pain that resolves only with stone passage or removal. The pain may migrate anteriorly along the abdomen and inferiorly to the groin, testicles, or labia majora as the stone moves toward the ureterovesical junction. Gross hematuria, urinary urgency, frequency, nausea, and vomiting may occur. Stones smaller than 5 mm usually pass spontaneously with hydration, whereas larger stones often require urologic intervention (Fig. 59-2).14,16 Ureteral colic may also occur with the passage of clots from hematuria of any cause (“clot colic”), or with papillary necrosis. As well as colic, nephrolithiasis may provoke less-specific loin pain that poorly localizes to the kidney and therefore has a wide differential diagnosis, particularly if not associated with other urinary symptoms. The finding of a stone on radiologic examination does not preclude a coincidental cause of pain from another source.
Hematuria
Stone disease is a common cause of hematuria. Macrohematuria occurs more commonly with large calculi and during UTI and colic. Although typically associated with loin pain or ureteral colic, the hematuria of nephrolithiasis may also be painless. The clinical differential diagnosis of hematuria is therefore wide (Box 59-1). Painless microhematuria in children may occur with hypercalciuria in the absence of demonstrable stones.
Loin Pain–Hematuria Syndrome
Loin pain–hematuria syndrome is a poorly understood condition that must always be considered in the differential diagnosis of nephrolithiasis. It is diagnosed by exclusion when patients (most typically young and middle-aged women) present with loin pain and persistent microhematuria or intermittent macrohematuria.17,18 Careful evaluation is required to exclude small stones, tumor, UTI, and glomerular disease. Angiographic abnormalities implying intrarenal vasospasm or occlusion have been reported, as have renal biopsy abnormalities typified by deposition of complement C3 in arteriolar walls. However, these findings are not consistent, nor do they provide a coherent framework to explain the pathogenesis of this condition.
In one study, 43 consecutive patients with clinical manifestations of loin pain–hematuria syndrome were evaluated by renal biopsy after other causes of their symptoms had been excluded with at least two imaging studies.17 Thirty-four patients were considered to have idiopathic loin pain–hematuria syndrome after nine with histologic evidence of IgA nephropathy were excluded. Of these, 66% had glomerular basement membranes that were either unusually thick or thin on electron microscopy, and 47% had a history of kidney stones, though none had obstructing stones at the time of assessment. Evidence of glomerular hematuria was more common in biopsies of patients with loin pain–hematuria syndrome compared with those of healthy living kidney donors who also underwent renal biopsy. The investigators postulated that the structurally abnormal glomerular basements in the majority of these patients may lead to rupture of the glomerular capillary walls, with consequent hemorrhage into the renal tubules. Tubular obstruction by red blood cells or potentially by microcrystals ensues. Local and global renal parenchymal edema follow, ultimately resulting in stretching of the renal capsule and in severe flank pain.
Loin pain–hematuria syndrome is a chronic condition requiring reassurance, careful management of analgesia, and ongoing psychological support. The condition usually remits after several years. Denervation of the kidney by autotransplantation is rarely successful.18 The extreme measure of nephrectomy has been used, but pain often recurs promptly in the contralateral kidney. Bilateral nephrectomy and renal replacement therapy has been reported as an approach of very last resort. Referral to a pain clinic can assist in providing psychiatric counseling, analgesia, and exclusion of other disorders. In one retrospective study, patients who eventually came to accept a nonsurgical approach along with pain-coping strategies that did not involve narcotic analgesics had the most successful outcomes.19
Asymptomatic Stone Disease
Even large staghorn calculi may be asymptomatic and discovered only during the investigation of unrelated abdominal or musculoskeletal symptoms. Obstructive uropathy caused by calculi may also be painless; therefore, nephrolithiasis should always be considered in the differential diagnosis of unexplained renal failure.
In the outbreak of melamine-associated nephrolithiasis in Chinese infants, the majority of patients brought to a screening clinic had no symptoms or signs of stones, and the diagnosis of nephrolithiasis was made only by ultrasound in at-risk infants and toddlers.12
Clinical Evaluation of Stone Formers
All patients with recurrent nephrolithiasis merit metabolic evaluation to determine the cause of their kidney stones. Complete evaluation of patients who have formed only a single stone is controversial because of the undetermined cost-benefit ratio. A National Institutes of Health Consensus Development Conference on the Prevention and Treatment of Kidney Stones determined that all patients, even those with a single stone, should undergo a basic evaluation, which need not include a 24-hour urine collection. Those with metabolically active stones (stones growing in size or number within 1 year), all children, noncalcium stone formers, and patients in demographic groups not typically prone to stone formation warrant a more complete evaluation, which includes a 24-hour urine collection made with the patient taking his or her typical diet.20
Basic Evaluation
The clinic evaluation of stone formers includes a general history and physical examination and requires specific data gathering on stone formation, diet, and specific laboratory studies, as shown in Box 59-2.
History
The history serves to uncover a systemic cause for nephrolithiasis. Any disease that can lead to hypercalcemia (including malignancy, hyperparathyroidism, and sarcoidosis) can result in hypercalciuria and increase the risk for calcium stone formation. A number of malabsorptive gastrointestinal disorders (including Crohn disease and sprue [celiac disease]) can result in calcium oxalate stone formation as a result of volume depletion and hyperoxaluria. Uric acid stones often occur in patients with a history of gout and, increasingly, in patients with insulin resistance.11
The stone history (see Box 59-2) includes the number and frequency of stones formed, patient age at incidence of first stone, size of stones, stone type (if known), and whether the patient required surgical removal of the calculi. This information indicates the severity of the stone disease and provides clues to the cause of the stone formation. For example, large staghorn calculi that do not pass spontaneously and recur despite frequent surgical intervention are more consistent with struvite than calcium oxalate stones. Stones that develop at a young age may be caused by cystinuria or primary hyperoxaluria. Stone response to intervention is also significant; cystine stones, for example, do not fragment well with lithotripsy. If stones recur frequently in a single kidney, a congenital abnormality in that kidney, such as megacalyx or medullary sponge kidney, should be explored.
Family history is important because a number of stone types have a genetic basis. Idiopathic hypercalciuria is most likely a polygenic disorder. Mutations in the claudins, which regulate calcium reabsorption in the thick ascending limb of the loop of Henle, cause familial hypercalciuria and nephrocalcinosis. A genome-wide association study in kidney stone patients identified sequence variants in the gene encoding claudin 14 that were associated with hypercalciuria.21
Cystinuria is usually autosomal recessive, and hyperuricosuria has been associated with rare inherited metabolic disorders. Nephrolithiasis and nephrocalcinosis can also result from a variety of monogenic disorders, such as Dent disease (X-linked recessive nephrolithiasis), McCune-Albright syndrome, osteogenesis imperfecta type 1, and congenital lactate deficiency. The various genetic disorders can lead to hypercalciuria by increasing bone resorption, affecting intestinal absorption, or decreasing renal tubular reabsorption transport, or through other, as yet unknown mechanisms.4,14,15,22,23
A number of medications are known to potentiate calcium stone formation (e.g., loop diuretics are calciuric) or may predispose to uric acid lithiasis (salicylates, probenecid) (Box 59-3). Certain drugs can precipitate into stones themselves, such as rapidly infused intravenous acyclovir, high-dose sulfadiazine, triamterene, and the antiretroviral agents indinavir and nelfinavir.24 In addition, some medications, such as acetazolamide and topiramate (a medication used for seizures and migraine headaches), promote nephrolithiasis by inhibiting carbonic anhydrase activity. In this setting the metabolic acidosis that ensues, along with lower urine citrate, higher urine pH, and increased urinary calcium excretion, predisposes to calcium phosphate stone formation.25
The social history should include details regarding occupation and lifestyle. Cardiothoracic surgeons and real-estate agents, for example, may minimize fluid intake to avoid bathroom breaks during the workday. Those who engage in vigorous physical activities, such as running, may not rehydrate adequately to keep up with insensible losses, producing excessively concentrated urine and precipitation of stone crystals in those prone to nephrolithiasis.
A dietary history and review of fluid intake are essential in determining potential causes or contributors to stone formation. The patient should be asked about commonly consumed foods, with attention paid to sodium-containing foods, as well as quantities of calcium, animal protein, purine, and oxalate (Box 59-4). Dietary calcium intake should be reviewed, because many patients with nephrolithiasis are erroneously instructed to eliminate all calcium from their diet, a suggestion that can result not only in bone demineralization, particularly in women and children, but also in an increase in stone formation.1–3,26–28 Sugar-sweetened soda appears to be associated with a greater risk of stone formation, whereas consumption of coffee, tea, beer, wine, and orange juice appears to be associated with a lower risk.29 The exact mechanism of nephrolithiasis from soda is not known but is likely related to the high fructose content of soda which may alter urinary composition and pH.30
Physical Examination
Most patients with idiopathic hypercalciuria are healthy and have normal physical examination findings. Patients with hyperuricosuria and uric acid stone formation may display tophi. Central obesity is associated with a predisposition to metabolic syndrome and uric acid stones. Paraplegic patients with a chronic indwelling bladder catheter may be predisposed to chronic UTI and struvite stones.
Laboratory Findings
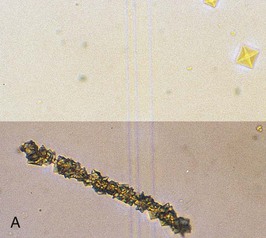
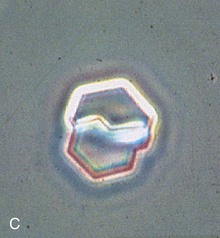
Urine pH is generally high in patients with struvite and calcium phosphate stones but low in patients with uric acid and calcium oxalate stones. The specific gravity, if high, will confirm inadequate fluid intake in many patients. Hematuria may imply active stone disease with crystal or stone passage. Examination of the urine may reveal red blood cells along with characteristic crystals (Fig. 59-3). Bacteriuria with urine pH of 6 to 6.5 suggests struvite stones. Urine should be cultured, and because many bacteria produce urease even when urine bacterial colony counts are low, the microbiology laboratory should be instructed to type the organism even if there are fewer than 100,000 colony-forming units/ml.
Blood tests required in the basic evaluation are serum electrolytes (sodium, potassium, chloride, and bicarbonate), creatinine, calcium, phosphorus, and uric acid. If the serum calcium is elevated or at the upper limit of normal, especially if the serum phosphorus is low, a serum parathyroid hormone level should be measured. A low potassium or bicarbonate level may indicate a cause for hypocitraturia, such as distal renal tubular acidosis.
Stone Analysis
Patients should be encouraged to retrieve any stone they excrete for chemical analysis, which may help define the underlying metabolic abnormality and guide therapy.
Imaging
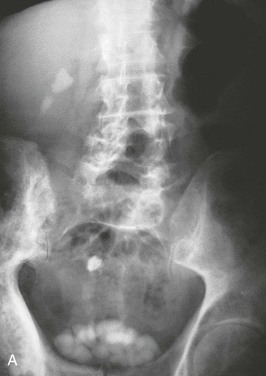
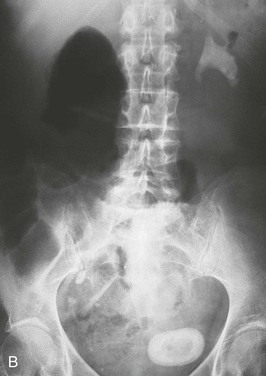
Patients should have a plain film of the abdomen performed with views of the kidneys, ureters, and bladder (KUB). This may reveal opacifications in the areas of the kidneys and ureters that could be a result of calcium, cystine, or struvite stones (Fig. 59-4). Uric acid and xanthine calculi are radiolucent and will not be visible on plain films. The unenhanced helical computed tomography (CT) scan, also known as spiral CT, or CT urography, has replaced contrast intravenous urography (IVU) as the diagnostic test of choice for acute ureteral colic; it has a higher sensitivity and specificity than IVU for detecting ureteral stones and ureteral obstruction16 and avoids the need for contrast. CT scanning is more likely to reveal causes of colic other than stones. It is more rapid, with results being available in minutes rather than hours, an advantage in the emergency room setting. However, an experienced radiologist, required for optimal interpretation of the images, may not be available at all times in urgent care facilities. Disadvantages of CT imaging include a radiation dose approximately three times that of a conventional IVU, and higher cost. Both tests should be avoided or limited in patients at risk for radiation exposure such as children and pregnant women.31,32 The role of CT urography is also discussed in Chapter 5.
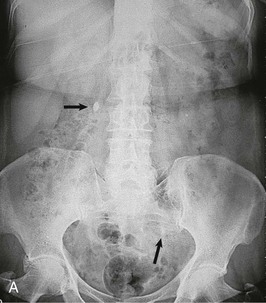
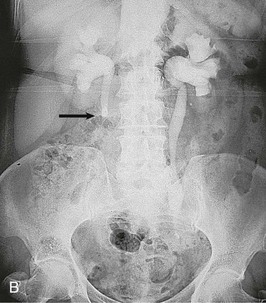
Contrast should generally be avoided in patients with renal impairment or other contraindications to the use of contrast. The IVU generally demonstrates urinary tract obstruction caused by calculi and can identify abnormalities of the genitourinary tract that may predispose to stone formation, such as medullary sponge kidney (see Chapter 47) or calyceal anomalies (see Chapter 52) (Fig. 59-5). During acute colic, the radiographic contrast used in the IVU, by creating a strong osmotic diuresis, may assist in moving the stone along the ureter. If stones are radiopaque, a KUB can be obtained if a patient develops symptoms suggestive of recurrent stone disease.
Renal ultrasound provides great specificity in the evaluation of stones, but is not a sensitive screening test. Both radiolucent and radiopaque stones within the kidneys should be detectable on ultrasound, but ureteral stones are often missed. Nonetheless, this is the test of choice for those patients who must avoid radiation exposure.
Periodic monitoring, if deemed necessary, should be obtained with a KUB and/or ultrasound scan rather than CT, whenever possible, to minimize radiation exposure. The combination of ultrasound and KUB has been found to be more sensitive in detecting stones than either test alone, while minimizing radiation compared with CT.33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree